Cancer of unknown primary
The optimal cancer care pathway is intended to guide the delivery of consistent, safe, high-quality, evidence-based care for people with cancer.
The pathway aligns with key service improvement priorities, including providing access to coordinated multidisciplinary care and supportive care and reducing unwanted variation in practice.
The optimal care pathway can be used by health services and professionals as a tool to identify gaps in current cancer services and to inform quality improvement initiatives across all aspects of the care pathway. The pathway can also be used by clinicians as an information resource and tool to promote discussion and collaboration between health professionals and people affected by cancer.
The following key principles of care underpin the optimal care pathway.
Patient-centred care
Patient- or consumer-centred care is health care that is respectful of, and responsive to, the preferences, needs and values of patients and consumers. Patient- or consumer-centred care is increasingly being recognised as a dimension of high-quality health care in its own right, and there is strong evidence that a patient-centred focus can lead to improvements in health care quality and outcomes by increasing safety and cost-effectiveness as well as patient, family and staff satisfaction (ACSQHC 2013).
Safe and quality care
Safe and quality care is provided by appropriately trained and credentialled clinicians, hospitals and clinics that have the equipment and staffing capacity to support safe and high-quality care.
It incorporates collecting and evaluating treatment and outcome data to improve the patient experience of care as well as mechanisms for ongoing service evaluation and development to ensure practice remains current and informed by evidence. Services should routinely be collecting relevant minimum datasets to support benchmarking, quality care and service improvement.
Multidisciplinary care
Multidisciplinary care is an integrated team approach to health care in which medical and allied health professionals consider all relevant treatment options and collaboratively develop an individual treatment and care plan for each patient. There is increasing evidence that multidisciplinary care improves patient outcomes.
The benefits of adopting a multidisciplinary approach include:
- improving patient care through developing an agreed treatment plan
- providing best practice through adopting evidence-based guidelines
- improving patient satisfaction with treatment
- improving the mental wellbeing of patients
- improving access to possible clinical trials of new therapies
- increasing the timeliness of appropriate consultations and surgery and a shorter timeframe from diagnosis to treatment
- increasing access to timely supportive and palliative care
- streamlining pathways
- minimising duplication of services (Department of Health 2007a).
Supportive care
Supportive care is an umbrella term used to refer to services, both generalist and specialist, that may be required by those affected by cancer. Supportive care addresses a wide range of needs across the continuum of care and is increasingly seen as a core component of evidence-based clinical care. Palliative care can be part of supportive care processes. Supportive care in cancer refers to the following five domains:
- physical needs
- psychological needs
- social needs
- information needs
- spiritual needs.
All members of the multidisciplinary team (MDT) have a role in providing supportive care. In addition, support from family, friends, support groups, volunteers and other community-based organisations make an important contribution to supportive care.
An important step in providing supportive care is to identify, by routine and systematic screening (using a validated screening tool) of the patient and family, views on issues they require help with for optimal health and quality-of-life outcomes. This should occur at key points along the care pathway, particularly at times of increased vulnerability including:
- initial presentation or diagnosis (the first three months)
- the beginning of treatment or a new phase of treatment
- change in treatment
- change in prognosis
- end of treatment
- survivorship
- recurrence
- change in or development of new symptoms
- palliative care
- end-of-life care.
Following each assessment, potential interventions need to be discussed with the patient and carer and a mutually agreed approach to multidisciplinary care and supportive care formulated (NICE 2004).
Common indicators in patients with these cancers that may require referral for support include:
- malnutrition (as identified using a validated malnutrition screening tool or presenting with weight loss)
- breathlessness
- pain
- difficulty managing fatigue
- difficulty sleeping
- distress, depression, anxiety or fear
- poor performance status
- living alone or being socially isolated
- having caring responsibilities for others
- cumulative stressful life events
- existing mental health issues
- Aboriginal or Torres Strait Islander status
- being from a culturally or linguistically diverse background.
Depending on the needs of the patient, referral to an appropriate health professional(s) and/or organisation(s) should be considered including:
- a clinical psychologist or psychiatrist
- a genetic counsellor
- community-based support services (such as Cancer Council Australia)
- a dietitian
- an exercise physiologist
- a nurse practitioner or specialist nurse
- an occupational therapist
- a physiotherapist
- peer support groups (contact the Cancer Council on 13 11 20 for more information)
- a social worker
- specialist palliative care
- a speech pathologist.
See the Appendix for more information on supportive care and the specific needs of people with cancer of unknown primary (CUP).
Care coordination
Care coordination is a comprehensive approach to achieving continuity of care for patients. This approach seeks to ensure that care is delivered in a logical, connected and timely manner so the medical and personal needs of the patient are met.
In the context of cancer, care coordination encompasses multiple aspects of care delivery including MDT meetings, supportive care screening/assessment, referral practices, data collection, development of common protocols, information provision and individual clinical treatment.
Improving care coordination is the responsibility of all health professionals involved in the care of patients and should therefore be considered in their practice. Enhancing continuity of care across the health sector requires a whole-of-system response – that is, that initiatives to address continuity of care occur at the health system, service, team and individual levels (Department of Health 2007b).
Communication
It is the responsibility of the healthcare system and all people within its employ to ensure the communication needs of patients, their families and carers are met. Every person with cancer will have different communication needs, including cultural and language differences. Communication with patients should be:
- timely
- individualised
- truthful and transparent
- consistent
- in plain language (avoiding complex medical terms and jargon)
- culturally sensitive
- active, interactive and proactive
- ongoing
- delivered in an appropriate setting and context
- inclusive of patients and their families (with the patient’s consent).
In communicating with patients, healthcare providers should:
- listen to patients and act on the information provided by them
- encourage patients to express individual concerns, needs and emotional states
- tailor information to meet the needs of the patient, their carer(s) and family
- use professionally trained interpreters when communicating with people from culturally and linguistically diverse backgrounds
- ensure the patient and/or their carer(s) and family have the opportunity to ask questions
- ensure the patient is not the conduit of information between areas of care (it is the provider’s and healthcare system’s responsibility to transfer information between areas of care)
- take responsibility for communication with the patient
- respond to questions in a way the patient understands
- enable all communication to be two-way.
Healthcare providers should also consider offering the patient a Question Prompt List (QPL) before their consultation, and recordings or written summaries of their consultations. QPL interventions are effective in improving the communication, psychological and cognitive outcomes of cancer patients (Brandes et al. 2014). Providing recordings or summaries of key consultations may improve the patient’s recall of information and patient satisfaction (Pitkethly et al. 2008).
Research and clinical trials
Where practical, patients should be offered the opportunity to participate in research and/or clinical trials at any stage of the care pathway. Research and clinical trials play an important role in establishing efficacy and safety for a range of treatment interventions, as well as establishing the role of psychological, supportive and palliative care interventions (Sjoquist & Zalcberg 2013).
While individual patients may or may not receive a personal benefit from the intervention, there is evidence that outcomes for participants in research and clinical trials are generally improved, perhaps due to the rigour of the process required by the trial. Leading cancer agencies often recommend participation in research and clinical trials as an important part of patient care. Even in the absence of a measurable benefit to patients, participating in research and clinical trials will contribute to the care of cancer patients in the future (Peppercorn et al. 2004).
Timeframes to treatment: Timeframes should be informed by evidence-based guidelines (where they exist) while recognising that shorter timelines for appropriate consultations and treatment can reduce people’s distress. The following recommended timeframes are based on expert advice from the Cancer of Unknown Primary Working Group.
| Step in pathway | Care point | Timeframe |
| Presentation, initial investigations and referral | 2.3 Referral | Patients should be seen by a specialist within two weeks of a referral from their general practitioner. |
| Diagnosis, staging and treatment planning | 3.1 Diagnostic work-up | Investigations should be completed within two weeks. |
| Treatment | 4.2 Treatment options | Treatment should start within two weeks of the
decision to treat. |
The optimal care pathway outlines seven critical steps in the patient journey. While the seven steps appear in a linear model, in practice, patient care does not always occur in this way but depends on the particular situation (such as the type of cancer, when and how the cancer is diagnosed, prognosis, management, patient decisions and the patient’s physiological response to treatment).
The pathway describes the optimal cancer care that should be provided at each step.
Special considerations
CUP is a distinct clinical entity. In Australia in 2014, CUP was the13th most commonly diagnosed cancer and in 2016 the sixth leading cause of cancer death. The number of new cases of CUP diagnosed in Australia increased from 2,141 in 1982 to 2,666 in 2014. Over the same time period, the age-standardised incidence rate decreased from 18 cases per 100,000 people in 1982 to 9.7 cases per 100,000 people in 2014 (AIHW 2018).
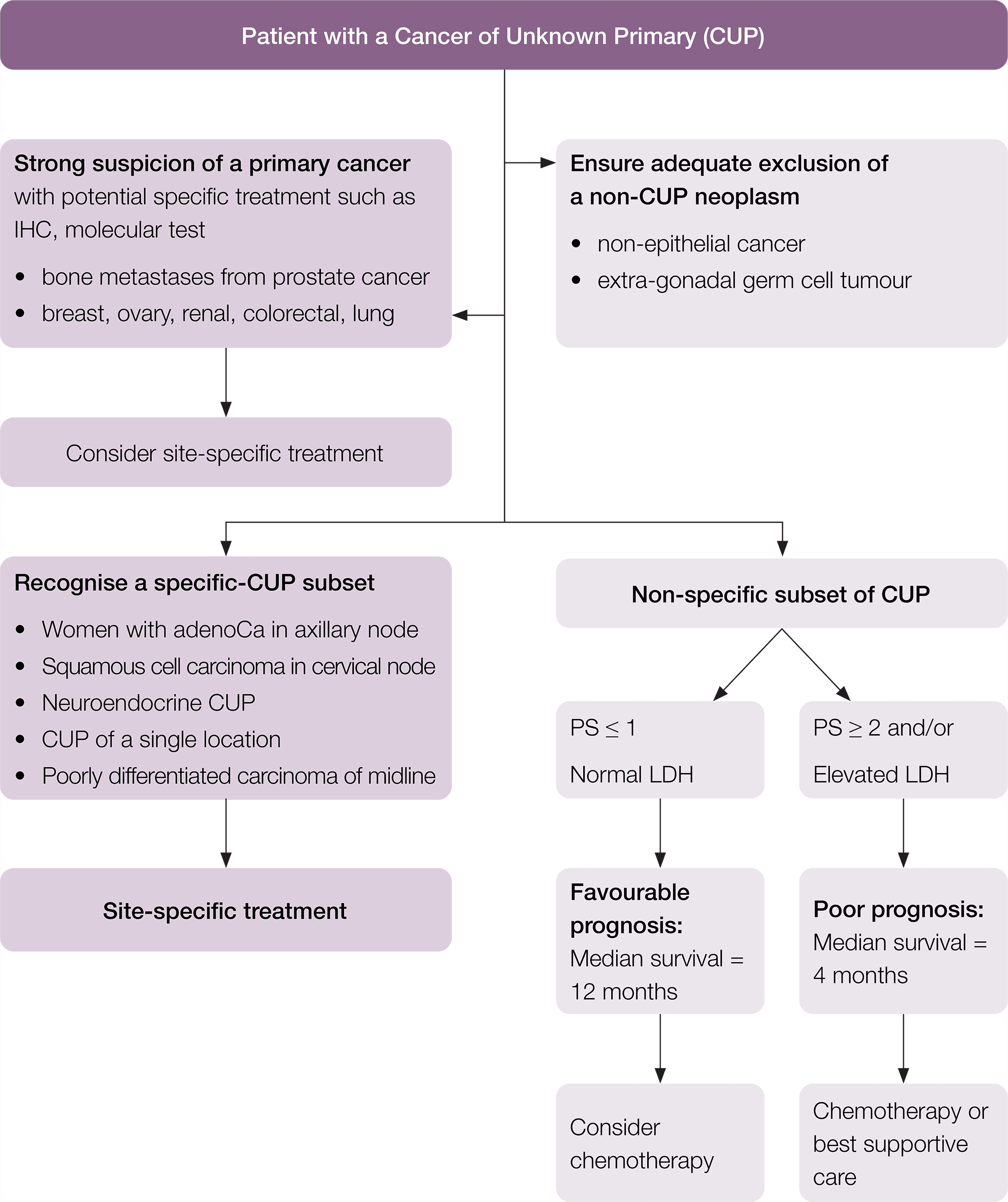
The term ‘cancer of unknown primary’ refers to a metastatic malignancy for which a standardised diagnostic work-up fails to identify the site of origin. CUP is a very heterogeneous disease in which the type of tumour, the extent of disease and the outcome of treatment all vary widely (NICE 2010). The entry point for this optimal care pathway is when patients present with a metastatic malignancy without an obvious primary site. Many of these patients will have a malignancy of epithelial lineage. The term CUP in this care pathway refers to patients with tumours of epithelial, neuroendocrine or undifferentiated lineage with no known primary site.
Malignancies of definitive non-epithelial lineage, such as melanoma, sarcoma and lymphoma, form a minority group of CUP where management can be undertaken without identifying a primary site; therefore, these malignancies are not specifically included in this pathway. Once a patient is diagnosed with melanoma, sarcoma or lymphoma, they should be treated according to existing tumour-specific pathways.
Diagnosing CUP is a continuum from initial presentation with the results of limited tests, to a final diagnosis after all relevant investigations have been completed. CUP may be suspected when a patient presents with metastatic malignancy on clinical examination or by imaging without an obvious primary site. CUP is confirmed when metastatic epithelial, neuroendocrine or undifferentiated malignancy is identified on the basis of final histology, where a reasonable amount of investigations have failed to identify a primary site with certainty (NICE 2010).
Some patients are given a label of ‘suspected CUP’ based on their clinical picture and radiological findings without any histopathological examination of the tumour having occurred because they may be assessed as being too unwell at presentation to undergo any form of further investigation or treatment. Hence the actual diagnosis of these patients is in fact unknown. These patients are still considered throughout this pathway and fit into the subset of patients with unfavourable prognosis as described below.
CUP subsets
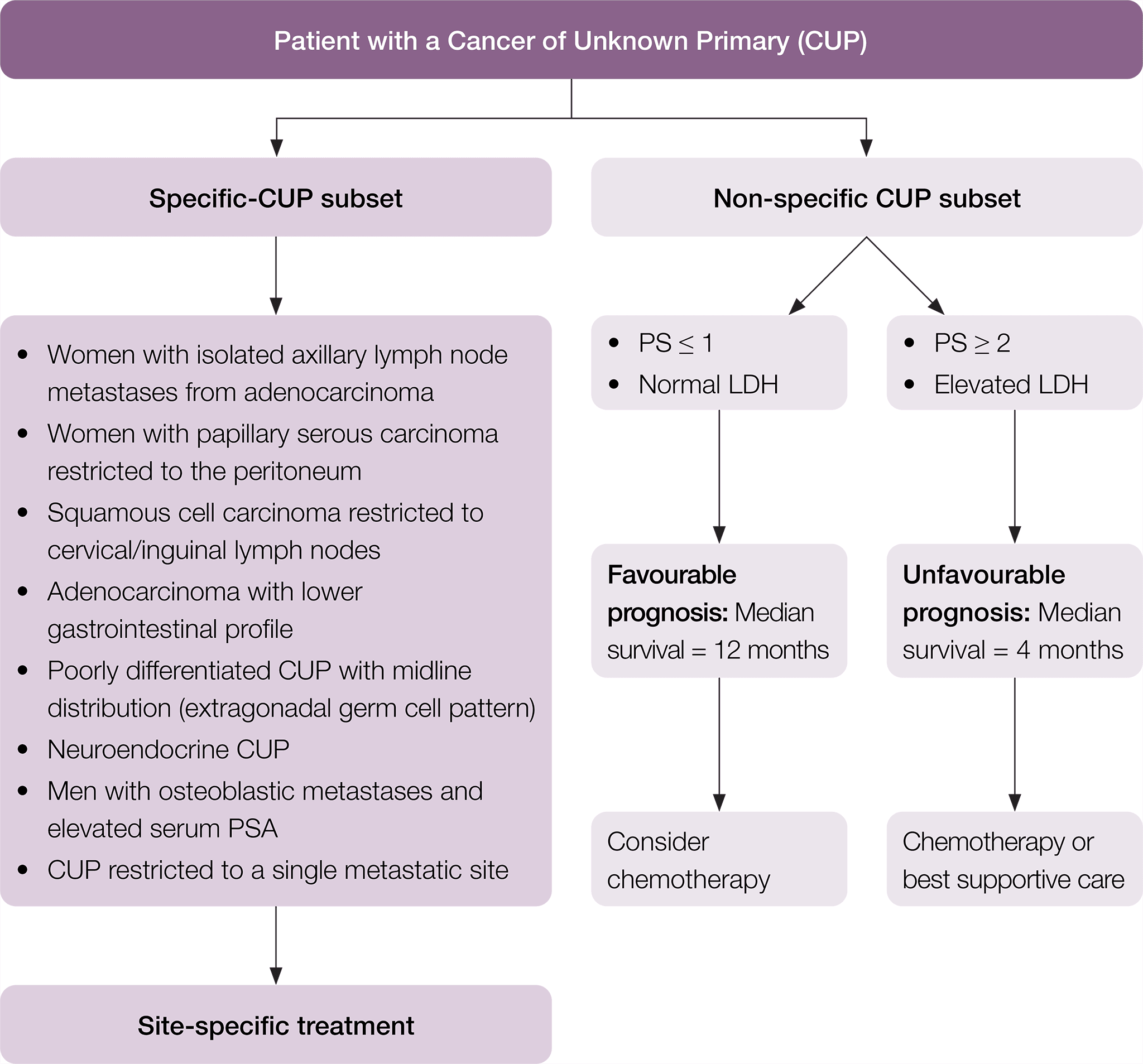
Among patients diagnosed with CUP, specific subsets can be identified based on histopathological and clinical manifestations (Pavlidis et al. 2015). In particular, there is a specific clinico-pathological subset of patients with favourable prognosis that have a pattern of disease similar to known cancer types and may respond well to standard disease-specific treatment. The remaining patients are considered within the non-specific subset of CUP, with their prognosis and suitability for treatment dependent on their Eastern Cooperative Oncology Group (ECOG) performance status and lactate dehydrogenase (LDH) level. Where appropriate to do so, this pathway has been divided into these subgroups of patients. In this pathway, the following definitions are used.
Specific clinico-pathological subsets of CUP include (Fizazi et al. 2015, Pavlidis & Fizazi 2005, Pavlidis & Pentheroudakis 2012, Vajdic & Goldstein 2015):
- poorly differentiated neuroendocrine carcinoma of unknown primary
- well-differentiated neuroendocrine tumour of unknown primary
- peritoneal adenocarcinomatosis of a serous papillary histological type in females
- isolated axillary nodal metastases in females
- squamous cell carcinoma involving non-supraclavicular cervical lymph nodes
- isolated inguinal adenopathy (squamous carcinoma)
- CUP with an intestinal phenotype and immunohistochemistry (IHC) (including CK20+/CDX2+/CK7−) or molecular profile
- single metastatic deposit from unknown primary
- men with blastic bone metastases or IHC/serum prostate specific antigen (PSA) expression
- poorly differentiated carcinoma with midline distribution (extragonadal germ cell syndrome).
Non-specific clinico-pathological subsets of CUP include patients with CUP who do not belong to one of the specific CUP subsets. Examples include (Pavlidis et al. 2015, Vajdic & Goldstein 2015):
- adenocarcinoma metastatic to the liver or other organs
- poorly differentiated carcinoma
- non-papillary malignant ascites (adenocarcinoma)
- multiple cerebral metastases (adeno or squamous carcinoma)
- multiple lung/pleural metastases (adenocarcinoma)
- multiple bone metastases (adenocarcinoma)
- squamous abdominopelvic CUP
- undifferentiated malignancy that cannot be further classified.
Non-specific CUP patients may be considered to be of (Fizazi et al. 2015):
- favourable prognosis if they have a good ECOG performance score (0–1) and a normal serum LDH (the median life expectancy is around one year)
- Unfavourable prognosis if they have either a poor ECOG performance score (≥ 2) and/or elevated serum LDH (the median life expectancy is around four months).
* Fizazi K, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2015; 26 (suppl_5): v133–v138 doi:10.1093/annonc/mdv305. Adapted and reproduced with permission of Oxford University Press on behalf of ESMO. Oxford University Press and ESMO are not responsible or in any way liable for the accuracy of the adaptation, for any errors, omissions or inaccuracies, or for any consequences arising therefore. Cancer Institute NSW is solely responsible for the adapted material in this work. Please visit the ESMO Cancers of Unknown Primary Site website
Not smoking, limiting sun exposure, eating a healthy diet, avoiding or limiting alcohol intake, taking regular exercise, maintaining a healthy body weight and avoiding exposure to oncoviruses or carcinogenic insults may help reduce cancer risk generally.
This step outlines recommendations for the prevention and early detection of CUP.
The causes of CUP are not fully understood, and there is currently no clear prevention strategy. However, general advice about ways to reduce cancer risk should be advised. Consideration should also be given to identifying individuals carrying a deleterious mutation conferring increased genetic risk. In some cases, CUP may be a manifestation of an undiagnosed hereditary cancer syndrome, with implications for the patient and their family. Patients with CUP with a significant family history of multiple cancers may suggest a heritable mutation within the family such as Lynch syndrome or a germline P53 or BRCA mutation. These patients should be referred to a familial cancer centre for further assessment.
As the primary cancer site is not known for people with CUP, it is difficult to identify risk factors. Evaluating risk is also challenging because of non-uniformity between CUP cases. CUP and advanced cancer research indicates the following factors may increase a person’s risk of developing CUP (AIHW 2018, Brewster et al. 2014, Kaaks et al. 2014, Luke et al. 2008, Urban et al. 2013):
- increasing age
- male gender
- family history of cancer
- smoking
- excessive alcohol consumption
- excess body fat
- inadequate physical activity
- living in areas of lower socioeconomic status
- Aboriginal and Torres Strait Islander background
- infrequent general practice consultations.
There is no population-based specific screening program for CUP.
This step outlines the process for establishing a diagnosis and appropriate referral. The types of investigation undertaken by the general practitioner (GP) depend on many factors, including access to diagnostic tests and medical specialists and patient preferences.
It is important for patients with CUP to be recognised as early as possible so that specialist assessment and management is not delayed and futile investigations are avoided.
In order to achieve this, a clear definition of CUP is required so that patients can be directed along a specialised pathway analogous to the pathways for existing tumour streams where possible.
Patients often present to their general or primary medical practitioner with heterogeneous, non- specific symptoms and abnormal test results that demonstrate very likely metastatic malignancy but without a clear primary site on history or initial investigations.
While some people may have no symptoms, common symptoms may include (Cancer Council Australia 2017, Vajdic & Goldstein 2015):
- loss of appetite
- fatigue
- weight loss
- breathlessness or discomfort in the chest
- cough
- persistent pain (for example, bone, back, abdomen)
- swelling of the abdomen
- jaundice
- swollen lymph glands
- headaches.
GP assessments will include routine tests for a patient with apparent or suspected metastatic disease, guided by the symptoms of the individual patient.
Assessments that should be undertaken by the GP for a patient with apparent or suspected metastatic disease include:
- a thorough medical history and physical examination, including systematic review to identify signs and symptoms that might suggest a possible primary site
- routine blood tests
- contrast-enhanced computed tomography (CT) of the chest/abdomen and pelvis
- arrangement of a biopsy in patients with readily accessible disease to allow early diagnosis of those with specific CUP subsets and established treatment pathways (Note: Consideration should be given as to whether this is best done by the GP or the specialist team, depending on the jurisdiction. In general, a core biopsy rather than a fine needle aspiration (FNA) is preferred to increase the chances of collecting sufficient material for a specific diagnosis (Berner et al. 2003))
- additional investigations as indicated based on the specific presentation, clinical symptoms and signs of the patient. (These are discussed in section 3.1). In some situations, if they can be easily obtained, it may be appropriate for the GP to organise some of these tests in parallel with specialist referral to expedite the work-up of the patient.)
Further important considerations
- Patients with CUP may present as clinically unwell with poorly controlled symptoms. Symptomatic care must be provided in parallel with the investigation process.
- Patients with suspected CUP and poor performance status are at risk of over-investigation, and results from exhaustive tests commonly will not result in useful action. Early specialist input should be sought to limit the extent of futile investigation.
- Early specialist input will also assist in dealing with the uncertainty that patients and their carers experience during the confusing period when clear answers to their diagnosis and prognosis are not apparent.
Patients with a disease pattern suggesting a specific CUP subset should be referred to a relevant disease-specific oncology team. These include patients with lymphadenopathy restricted to the neck, and a biopsy showing squamous cell carcinoma should be referred to a specialist on a head and neck cancer MDT. Women with isolated axillary nodal metastases should be referred to a specialist on a breast cancer MDT.
When apparent metastatic disease without a clear primary site is recognised as CUP, patients should be referred to an oncologist with adequate experience in managing acute patients with CUP. Oncology services should identify oncologists with an interest in CUP within their services where available, and should provide clear routes of rapid access to specialist evaluation.
Patients who appear to fall into a non-specific CUP subset may be referred to the general medical oncology service, which can then triage the patient to be seen in the most appropriate clinic depending on the jurisdiction. Since most centres do not have a specific CUP clinic, this may include the patient being seen in a general oncology clinic or a disease-specific clinic depending on the presentation and suspected diagnosis.
Referral for suspected CUP should incorporate appropriate documentation sent with the patient including:
- the patient’s name, date of birth, contact details and next of kin
- details of the patient’s guardian, advance care plan or enduring power of attorney if relevant
- a letter that includes important psychosocial history and relevant past history, family history, current symptoms, medications and allergies
- results of current clinical investigations (imaging and pathology reports)
- results of all prior relevant investigations
- any prior imaging, particularly a hard copy or CD of previous chest x-rays and CT scans where online access is not available (lack of a hard copy should not delay referral)
- notification if an interpreter service is required.
Although referral to specialist oncology services is essential, it is important that regular communication between the oncology specialists and the referring GP occurs to ensure timely, coordinated care and support for the patient and their family.
Timeframe for referral to a specialist
Timeframes for specialist referral should be informed by evidence-based guidelines (where they exist) while recognising that shorter timelines for appropriate consultations and treatment can reduce patient distress.
Patients with CUP should be referred to a specialist for further investigation. The specialist appointment should take place within two weeks of the initial GP referral. A patient with uncontrolled symptoms or who is deteriorating may require urgent assessment, and the GP should call the medical oncology unit to expedite a review.
An individualised clinical assessment is required to meet the identified needs of a patient, their carer(s) and family. Referral should be as required; however, given the generally poor prognosis of this malignancy, early supportive care is strongly encouraged.
In addition to common issues identified in the Appendix, specific needs that may arise at this time include:
- communication to ensure the patient understands that they are being investigated for a possible cancer diagnosis, even prior to a tissue diagnosis being obtained
- support and information to assist with any distress while patients are undergoing these tests – patients with CUP will most likely undergo extensive testing (Wagland et al. 2017)
- psychosocial support and specific interventions – depressive symptoms are higher in people with CUP when compared with people with cancer of a known origin (Hyphantis et al. 2013)
- good communication from experts – CUP patients are more likely to want written information about their type of cancer and tests received but less likely to understand explanations of their condition (Wagland et al. 2017)
- coordination of care – GPs play an important role in coordinating care for patients, including assisting with side effects and offering support when questions or worries arise
- treatment for physical symptoms such as pain and fatigue
- guidance about financial and employment issues (such as loss of income, travel and accommodation requirements for rural patients and caring arrangements for other family members)
- appropriate information for people from culturally and linguistically diverse backgrounds.
Effective communication is essential at every step of the care pathway. Effective communication with the patient and carer is particularly important given the prevalence of low health literacy in Australia, which is estimated at 60 per cent of Australian adults (ACSQHC 2013).
The general or primary medical practitioner who made the referral is responsible for the person until care is passed to another practitioner.
The general or primary medical practitioner may play a number of roles in all stages of the cancer pathway including diagnosis, referral, treatment, coordination and continuity of care as well as providing information and support to the person and their family.
The GP should:
- provide the patient with information that clearly describes who they are being referred to, the reason for referral and the expected timeframe for appointments
- support the patient while waiting for the specialist appointment.
Step 3 outlines the process for confirming the diagnosis and planning subsequent treatment. The guiding principle is that interaction between appropriate MDT members should determine the treatment plan.
Diagnosis for CUP is continually evolving in all areas, including imaging, pathology and genetics. Every effort should be made to use current best practice evidence when confirming a diagnosis of CUP.
Optimal diagnostic workup should be quickly applied. It should aim to identify the primary tumours where possible. However, it is important that the risk of over-investigation is minimised in patients with poor performance status where exhaustive testing is unlikely to improve outcome. This needs to be balanced against the risk of missing treatable cancers.
Diagnostic workup for patients with metastatic disease should include all of the following investigations in order to confirm a diagnosis of CUP (adapted from Fizazi et al. 2015):
- a thorough medical history and physical examination including breast exam and rectal exam where clinically appropriate
- basic blood and biochemical analyses (full blood examination; LDH; electrolytes, urea, creatinine; liver function tests) – it is not of proven value to order tumour markers indiscriminately in the work-up of CUP; however, specific tumour markers may be of value depending on the clinical presentation
- contrast-enhanced CT scans of thorax, abdomen and pelvis
- adequate tissue sample from one site of disease for histopathology and IHC.
Additional investigations for identified CUP subsets:
- female patients –> mammography
- females with axillary adenocarcinoma without a breast primary on mammogram or breast ultrasound –> breast magnetic resonance imaging (MRI)
- people with midline metastatic disease –> serum α-fetoprotein and human chorionic gonadotropin
- signs and symptoms or laboratory abnormalities suggestive of a gastrointestinal primary –> endoscopies, carcinoembryonic antigen and CA19.9
- neuroendocrine tumours –> dotatate gallium / positron emission tomography (PET) scan and plasma chromogranin A
- squamous cell carcinoma restricted to neck nodes –> head and neck PET/CT scan, fibre optic nasendoscopy
- men with bone metastases –> serum PSA
- based on signs and symptoms or laboratory abnormalities –> additional diagnostic pathology such as specific tumour markers as directed by the clinical pattern of disease.
Note: The role of PET/CT in extracervical CUP is controversial. The literature suggests that a primary may be identified in 30–40 per cent of patients, but the impact on patient outcomes with the routine use of PET in CUP cases is unclear (Burglin et al. 2017), and there is currently no specific Medicare rebate available for PET/CT in CUP. However, the use of PET/CT may be appropriate in patients with apparent localised CUP who are being considered for treatment with curative intent.
In patients with a significant family or personal history of cancer (see eviQ guidelines), consideration should be given to referral for genetic testing.
Biopsy material should be examined by an anatomical pathologist with expertise in this area, and may require referral for a second opinion. The use of specific IHC panels is promising in assigning a likely primary site of origin (Park et al. 2007). The choice of which IHC stains or ancillary pathology investigations to perform is highly dependent on the clinical setting, the morphology of the malignant cells and the available material in biopsy specimens. Therefore, no one set of IHC stains or ancillary tests is appropriate for all patients. The 20 cytokeratin (CK) subtypes are typically expressed in carcinomas. A CK7 plus CK20 staining pattern may lead to additional IHC staining and specific clinical tests, so these should be considered for all CUPs (Losa et al. 2018).
Important principles to consider in selecting the appropriate ancillary testing to perform on tissue include the following:
- Tissue biopsy is usually better than FNA because it provides more tissue for ancillary testing, including IHC.
- It is important to identify highly treatable non-epithelial malignancies such as lymphoma, melanoma and germ cell tumour.
- The need to optimally classify CUP with extensive ancillary testing that may exhaust tissue needs to be balanced against the limited tissue available; therefore, some testing may need to be triaged.
Examples where ancillary molecular testing may be appropriate include in patients where there is a strong clinical suspicion of a specific tumour type, where standard molecular profiling to look for actionable genomic abnormalities for that tumour type should occur. For example, patients with TTF-1 adenocarcinoma thought most likely to be lung cancer (even in the absence of an identifiable lung primary) should have testing for epidermal growth factor receptor mutations and, if this is negative, anaplastic lymphoma kinase re-arrangements.
The optimal role of molecular profiling tests to determine the tissue of origin is the subject of ongoing research. Existing guidelines do not recommend the use of gene-expression-based profiling to identify primary tumours. However, where this is available via CUP research projects, it may be helpful.
The use of gene panel DNA sequencing to identify actionable mutations is still under investigation. It appears that, in a minority of patients, potentially actionable mutations may be found (Gatalica et al. 2014, Ross et al. 2015, Tothill et al. 2013, Varghese et al. 2017). In addition, other findings such as mutational signatures indicating tobacco or UV exposure may assist in diagnosing the primary site (Tothill et al. 2013, Varghese et al. 2017). The use of immunotherapy may be effective in some patients, especially those with high tumour mutation burden (Gröschel et al. 2016). Panel testing may also identify germline risk alleles. There is currently a lack of evidence as to whether patients receiving this panel testing have an outcome benefit. However, where this is available via CUP research projects, it may be helpful.
Timeframe for obtaining a diagnosis
Timeframes for diagnosis should be informed by evidence-based guidelines (where they exist) while recognising that shorter timelines for appropriate consultations and treatment can reduce patient distress.
Investigations should be completed within two weeks.
People with CUP have a malignancy that has already spread; therefore, by definition it is a metastatic cancer. There is no definite staging classification used for patients with CUP; however, disease can be classified as localised or disseminated disease.
CUP is a very heterogeneous disease. There is a specific CUP subset of patients who have a pattern of disease similar to known cancers types that may respond well to standard disease-specific treatment. The remaining patients are considered within the non-specific subset of CUP, with their prognosis and suitability for treatment dependent on their ECOG performance status and LDH level.
Between 2010 and 2014, people with CUP had a 14 per cent chance of surviving for five years when compared with the age- and sex-matched general Australian population (AIHW 2018).
Patients in the specific-CUP subset should be discussed at the MDT meetings of the tumour stream most closely related to the person’s CUP.
Patients who appear to fall into a non-specific CUP subset should be referred to a CUP-specific oncology service or else to the general medical oncology service, which can then triage them into the most appropriate clinic for ongoing care depending on the jurisdiction. Since most centres do not have a specific CUP clinic this may include the patient being seen in a general oncology clinic or a disease-specific clinic depending on the presentation and suspected diagnosis.
The complex nature of CUP, along with the advantages gained from multidisciplinary collaboration in this situation, argue strongly for establishing dedicated MDT services for this group, even if convened via tele- or video-conferencing. However, where CUP MDTs do not exist, an MDT with members who have expertise in managing CUP should discuss the patient.
The responsibilities of the MDT are to:
- nominate a team member to coordinate patient care and identify this person to the patient
- nominate a team member to be the lead clinician (the lead clinician may change over time depending on the stage of the care pathway and where care is being provided) and identify this person to the patient (if different from the care coordinator)
- develop and document an agreed treatment plan at the MDT meeting
- communicate/circulate the agreed MDT treatment plan to relevant team members, including the patient’s GP.
The general or primary medical practitioner who made the referral is responsible for the patient until care is passed to another practitioner.
The general or primary medical practitioner may play a number of roles in all stages of the cancer pathway including diagnosis, referral, treatment and coordination and continuity of care as well as providing information and support to the patient and their family.
The care coordinator is responsible for ensuring there is continuity throughout the care process and for coordinating all necessary care for a particular phase. The care coordinator may change over the course of the pathway.
The lead clinician is responsible for overseeing the activity of the team.
The MDT should comprise the core disciplines that are integral to providing good care. Team membership will vary according to cancer type but should reflect both clinical and psychosocial aspects of care. Additional expertise or specialist services may be required for some patients (Department of Health 2007a).
Team members may include a:
- care coordinator (as determined by MDT members)*
- medical oncologist*
- pathologist*
- radiologist*
- surgeon*
- nurse (with appropriate expertise)*
- radiation oncologist*
- social worker*
- clinical trials coordinator
- dietitian
- GP
- psychologist
- nuclear medicine physician
- occupational therapist
- specialist palliative care team member(s)
- pharmacist
- physiotherapist
- psychiatrist
- rehabilitation physician
- speech therapist.
* Core members of the MDT are expected to attend most MDT meetings either in person or remotely.
Treatment options for all newly diagnosed patients should be discussed in an MDT meeting before beginning treatment. The level of discussion may vary depending on both the clinical and psychosocial factors.
There may also need to be a review of existing treatment plans for patients who have been discussed previously.
Results of all relevant tests and imaging should be available for the MDT discussion. The care coordinator or treating clinician should also present information about the patient’s concerns, preferences and social circumstances at the meeting (Department of Health 2007a).
Participation in research and/or clinical trials should be encouraged where available and appropriate.
- Australian Cancer Trials is a national clinical trials database. It provides information on the latest clinical trials in cancer care, including trials that are recruiting new participants. For more information visit the Australian Cancer Trials website.
Cancer prehabilitation uses a multidisciplinary approach combining exercise, nutrition and psychological strategies to prepare patients for the challenges of cancer treatment such as chemotherapy, immunotherapy and radiation therapy.
Evidence indicates that prehabilitation of newly diagnosed cancer patients prior to starting treatment can be beneficial. This may include conducting a physical and psychological assessment to establish a baseline function level, identifying impairments and providing targeted interventions to improve the patient’s health, thereby reducing the incidence and severity of current and future impairments related to cancer and its treatment (Silver & Baima 2013).
Medications should be reviewed at this point to ensure optimisation and to improve adherence to medicines used for comorbid conditions.
Where appropriate, fertility issues should be reviewed with the patient.
Screening with a validated screening tool (such as the National Comprehensive Cancer Network Distress Thermometer and Problem Checklist), assessment and referral to appropriate health professionals or organisations is required to meet the identified needs of the patient, their carer(s) and family.
In addition to the other common issues outlined in the Appendix, specific needs that may arise at this time include the following:
Physical
- Fatigue/change in functional abilities is a common symptom, and patients may benefit from referral to occupational therapy.
Psychological
- Many patients with CUP find the uncertainty surrounding their disease and the limited treatment options difficult and would welcome the opportunity to ask questions and learn about others’ experiences (Boyland & Davis 2008).
- Many patients with CUP and their clinicians have a poor understanding of their illness, difficulty in explaining their illness to others, and a sense of frustration in health professionals not having the answers (Boyland & Davis 2008, Karapetis et al. 2017).
- Depressive symptoms are higher in people with CUP when compared with people with cancer of a known origin, so they require more psychosocial support and specific interventions (Hyphantis et al. 2013).
- Information about genomic profiling available via CUP research projects should be provided to patients.
- GPs play an important role in coordinating care for patients, including assisting with side effects and offering support when questions or worries arise. For most patients, simultaneous care provided by their GP is very important (Lang et al. 2017).
- Patients may require help with psychological and emotional distress while adjusting to the diagnosis, treatment phobias, existential concerns, stress, difficulties making treatment decisions, anxiety/depression, loss of previous life roles including driving, and interpersonal problems.
Social/practical
- Patients may need support to attend appointments.
- Patients may need guidance about financial and employment issues (such as loss of income and having to deal with travel and accommodation requirements for rural patients and caring arrangements for other family members).
Information
- Patients with CUP will most likely undergo extensive testing, and support and information should be provided to assist with any distress while undergoing these tests (Wagland et al. 2017).
- CUP patients are more likely to want more written information about their type of cancer and tests received but less likely to understand explanations of their condition (Wagland et al. 2017).
- Patients from culturally and linguistically diverse backgrounds may need information provided in other formats.
- Patients may need advice about safe driving.
Spiritual needs
- Lead clinicians should have access to suitably qualified, authorised and appointed spiritual caregivers who can act as a resource for patients, carers and staff.
- Patients and their families should have access to spiritual support appropriate to their needs throughout the cancer journey.
The lead clinician should:
- ensure the patient understands that they have been diagnosed with CUP and that they understand the ramifications of this diagnosis
- assist the patient in explaining their diagnosis to their family; audio recordings may be a helpful resource for patients (Pitkethly et al. 2008)
- establish if the patient has a regular or preferred GP
- discuss a timeframe for diagnosis and treatment with the patient and carer
- discuss the benefits of multidisciplinary care and make the patient aware that their health information will be available to the team for discussion at the MDT meeting
- offer individualised CUP information that meets the needs of the patient and carer (this may involve advice from health professionals as well as written and visual resources)
- offer advice on how to access information and support from websites, community and national cancer services and support groups for both patients and carers
- use a professionally trained interpreter to communicate with people from culturally or linguistically diverse backgrounds.
The lead clinician should:
- ensure regular and timely (within a week) communication with the person’s GP regarding the treatment plan and recommendations from MDT meetings
- notify the GP and family/carer if the person does not attend clinic appointments
- gather information from the GP including their perspective on the person (psychological issues, social issues and comorbidities) and locally available support services
- ask the person’s GP to contribute to developing a chronic disease and mental healthcare plan as required
- discuss management of shared care with the person’s GP
- invite the GP to participate in MDT meetings (consider using video or teleconferencing).
Step 4 provides an overview of the treatment options for CUP. For detailed information on treatment options refer to National Comprehensive Cancer Network (NCCN) guidelines.
Refer also to the ESMO CUP guidelines.
The intent of treatment can be defined as one of the following:
- curative
- anti-cancer therapy to improve quality of life and/or longevity without expectation of cure, or
- symptom palliation.
The morbidity and risks of treatment need to be balanced against the potential benefits. The advantages and disadvantages of each treatment and associated potential side effects should be discussed with the patient.
The lead clinician should discuss treatment intent and prognosis with the patient and their family/ carer before beginning treatment.
Advance care planning should be initiated with patients and their carers as there can be multiple benefits such as ensuring a person’s preferences are known and respected after the loss of decision-making capacity (AHMAC 2011).
The advantages and disadvantages of each treatment and associated potential side effects should be discussed with the patient and their carer/family.
Treatment should be individualised according to the clinico-pathological subset and the suspected primary site. The following treatment recommendations have been adapted from the ESMO guidelines (Fizazi et al. 2015).
A suggested flow chart to guide treatment is provided.
* Fizazi K, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2015; 26 (suppl_5): v133–v138 doi:10.1093/annonc/mdv305. Adapted and reproduced with permission of Oxford University Press on behalf of ESMO. Oxford University Press and ESMO are not responsible or in any way liable for the accuracy of the adaptation, for any errors, omissions or inaccuracies, or for any consequences arising therefore. Cancer Institute NSW is solely responsible for the adapted material in this work. Please visit the ESMO Cancers of Unknown Primary Site website.
Patients in the specific-CUP subset who have a good prognosis should be treated the same as patients with equivalent known primary tumours with metastatic disease, as shown in Table 1.
|
Equivalent known primary tumour |
Recommended treatment |
|
Poorly differentiated neuroendocrine carcinoma of unknown primary |
Treat as poorly differentiated neuroendocrine carcinomas with a known primary |
|
Well-differentiated neuroendocrine tumour of unknown primary |
Treat as well-differentiated neuroendocrine tumour of a known primary site |
|
Peritoneal adenocarcinomatosis of a serous papillary histological type in females |
Treat as ovarian cancer |
|
Isolated axillary nodal adenocarcinoma metastases in females |
Treat as breast cancer |
|
Squamous cell carcinoma involving non-supraclavicular cervical lymph nodes |
Treat as head and neck squamous cell cancer |
|
CUP with an intestinal phenotype and IHC (CK20+/CDX2+/CK7−) or molecular profile |
Treat as metastatic colorectal cancer |
|
Single metastatic deposit from unknown primary |
Treat as single metastases by resection or high-dose (ablative) radiotherapy depending on the location |
|
Osteoblastic bone metastases or IHC/serum PSA expression in men |
Treat as prostate cancer |
|
Patients with extragonadal germ cell syndrome |
Treat as poor-prognosis germ cell tumour (Greco 2013). |
|
Isolated inguinal adenopathy (squamous carcinoma) |
Local dissection with or without local radiotherapy (Pavlidis et al. 2015) |
Other tumour specific optimal care pathways can be found here.
* Fizazi K, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2015; 26 (suppl_5): v133–v138 doi:10.1093/annonc/mdv305. Adapted and reproduced with permission of Oxford University Press on behalf of ESMO. Oxford University Press and ESMO are not responsible or in any way liable for the accuracy of the adaptation, for any errors, omissions or inaccuracies, or for any consequences arising therefore. Cancer Institute NSW is solely responsible for the adapted material in this work. Please visit the ESMO Cancers of Unknown Primary Site website.
For patients with a non-specific subset of CUP, but who have a favourable prognosis, a two-drug chemotherapy regimen as per the NCCN or ESMO guidelines should be considered (Culine et al. 2003, Gross-Goupil et al. 2012, Hainsworth et al. 2010).
Patients with localised disease may be suitable for local therapies such as high-dose (ablative) radiotherapy (Janssen et al. 2014) or surgical excision.
CUP patients identified in the poor-prognosis non-specific group can be considered for treatment with low-toxicity, palliative, chemotherapy regimens and/or best supportive care (Fizazi et al. 2015).
Using palliative radiotherapy to relieve local symptoms should also be considered where appropriate (Rich & Mendenhall 2016, Tey et al. 2017). In addition, other palliative procedures to assist in symptom control may also be considered in specific situations such as video-assisted thoracoscopic surgery pleurodesis or PleurX (if available) for interventional pain relief.
Timeframe for commencing treatment
Timeframes for diagnosis should be informed by evidence-based guidelines (where they exist) while recognising that shorter timelines for appropriate consultations and treatment can reduce patient distress.
Treatment of CUP should begin within two weeks of the decision to treat (four weeks from referral).
Participation in research and/or clinical trials should be encouraged where available and appropriate.
- Australian Cancer Trials is a national clinical trials database. It provides information on the latest clinical trials in cancer care, including trials that are recruiting new participants. For more information visit the Australian Cancer Trials website.
The following training and experience is required of the appropriate specialist(s):
- Medical oncologists (RACP or equivalent) must have adequate training and experience with institutional credentialling and agreed scope of practice within this area (ACSQHC 2004).
- Nurses must have adequate training in chemotherapy administration and handling and disposal of cytotoxic waste.
- Chemotherapy should be prepared by a pharmacist with adequate training in chemotherapy medication, including dosing calculations according to protocols, formulations and/or preparation.
- In a setting where no medical oncologist is locally available, some components of less complex therapies may be delivered by a medical practitioner and/or nurse with training and experience with credentialling and agreed scope of practice within this area under the guidance of a medical oncologist. This should be in accordance with a detailed treatment plan or agreed protocol, and with communication as agreed with the medical oncologist or as clinically required.
- Radiation oncologist (FRANZCR or equivalent) with adequate training and experience that enables institutional credentialling and agreed scope of practice within this area (ACSQHC 2004) and who is also a core member of an oncology MDT.
These include:
- a clearly defined path to emergency care and advice after hours
- access to basic haematology and biochemistry testing
- cytotoxic drugs prepared in a pharmacy with appropriate facilities
- occupational health and safety guidelines regarding handling of cytotoxic drugs, including safe prescribing, preparation, dispensing, supplying, administering, storing, manufacturing, compounding and monitoring the effects of medicines (ACSQHC 2011)
- guidelines and protocols to deliver treatment safely (including dealing with extravasation of drugs)
- appropriate nursing and theatre resources to manage complex surgery
- 24-hour medical staff availability
- 24-hour operating room access
- specialist pathology
- in-house access to radiology
- an intensive care unit
- trained radiotherapy nurses, physicists and therapists
- access to CT/MRI scanning for simulation and planning
- mechanisms for coordinating combined therapy (chemotherapy and radiation therapy), especially where the facility is not collocated
- access to allied health, especially nutrition health and advice.
Unless favourable and treatable disease is defined, CUP represents a group of patients who have a very poor prognosis. It has been demonstrated that CUP patients receive less treatment and have poorer survival compared with those with cancer of known primary sites (Riihimaki et al.2013a, Schaffer et al. 2015). Many people with CUP may benefit from palliative treatments to relieve symptoms, improve quality of life and manage the uncertainty of their prognosis more effectively.
Palliative care interventions should be considered for all patients diagnosed with CUP (NCCN 2017). It is preferable for specialist palliative care to be initiated during the diagnostic stage, and for many patients this will remain the most important intervention during their illness (Abdallah et al. 2014).
Early referral to palliative care can improve the quality of life for people with cancer including improving the capacity of patients to sit comfortably with diagnostic and prognostic uncertainty and better management of physical and psychological symptoms (Haines 2011, Temel et al. 2010, Temel et al. 2017, Zimmermann et al. 2014). This is particularly true for poor-prognosis cancers (Cancer Council Australia 2017, Philip et al. 2013, Temel et al. 2010, Temel et al. 2017). Furthermore, palliative care has been associated with improved wellbeing for carers (Higginson & Evans 2010, Hudson et al. 2014).
Ensure carers and families receive information, support and guidance regarding their role according to their needs and wishes (Palliative Care Australia 2005).
The patient and carer should be encouraged to develop an advance care plan (AHMAC 2011).
Further information
Refer patients and carers to Palliative Care Australia.
The lead clinician should discuss the patient’s use (or intended use) of complementary or alternative therapies not prescribed by the MDT to identify any potential toxicity or drug interactions.
The lead clinician should request a comprehensive list of all complementary and alternative medicines being taken and explore the patient’s reason for using these therapies and the evidence base.
Many alternative therapies and some complementary therapies have not been assessed for efficacy or safety. Some have been studied and found to be harmful or ineffective.
Some complementary therapies may assist in some cases, and the treating team should be open to discussing the potential benefits for the patient.
If the patient expresses an interest in using complementary therapies, the lead clinician should consider referring them to health professionals within the MDT who have knowledge of complementary and alternative therapies (such as a clinical pharmacist, dietitian or psychologist) to help them reach an informed decision.
The lead clinician should assure patients who use complementary or alternative therapies that they can still access MDT reviews (NBCC & NCCI 2003) and encourage full disclosure about therapies being used (Cancer Australia 2010).
Further information
- See Cancer Australia’s position statement on complementary and alternative therapies.
- See the Clinical Oncology Society of Australia’s position statement.
Screening with a validated screening tool (such as the National Comprehensive Cancer Network Distress Thermometer and Problem Checklist), assessment and referral to appropriate health professionals and/or organisations is required to meet the needs of individual patients, their families and carers.
In addition to the common issues outlined in the Appendix, specific needs that may arise at this time include the following.
Physical needs
- Fatigue/change in functional abilities is a common symptom, and patients may benefit from a referral to occupational therapy.
- Decline in mobility and/or functional status may result from treatment.
- Assistance with managing complex medication regimens, multiple medications, assessment of side effects and assistance with difficulties swallowing medications may be required. Refer to a pharmacist if necessary.
Psychological needs
- Patients may need support with emotional and psychological issues including, but not limited to, body image concerns, fatigue, existential anxiety, treatment phobias, anxiety/depression, interpersonal problems and sexuality concerns.
- Many patients with CUP find the uncertainty surrounding their disease and the limited treatment options difficult and would welcome the opportunity to ask questions and learn about others’ experiences (Boyland & Davis 2008).
- Many patients with CUP and their clinicians have a poor understanding of their illness, have difficulty in explaining their illness to others, and develop a sense of frustration in health professionals not having the answers (Boyland & Davis 2008, Karapetis et al. 2017).
- Depressive symptoms are higher in people with CUP when compared with people with cancer of a known origin, so they require more psychosocial support and specific interventions (Hyphantis et al. 2013).
- Palliative care can improve physical symptom control and the quality of life for people with cancer including improving the capacity of patients to come to terms with diagnostic and prognostic uncertainty (Temel et al. 2017).
- GPs play an important role in coordinating care for patients, including assisting with side effects and offering support when questions or worries arise. For most patients, simultaneous care provided by their GP is very important (Lang et al. 2017).
Social/practical needs
- Patients may need support to attend appointments.
- Potential isolation from normal support networks, particularly for rural patients who are staying away from home for treatment and for patients with neuropsychiatric symptoms, can be an issue. Social isolation can also compound distress (Australian Cancer Network 2009).
- Financial issues related to loss of income and additional expenses as a result of illness and/or treatment may require support.
- Help with legal issues may be required including advance care planning, appointing a power of attorney and completing a will.
Spiritual needs
- Multidisciplinary teams should have access to suitably qualified, authorised and appointed spiritual caregivers who can act as a resource for patients, carers and staff.
- Patients with cancer and their families should have access to spiritual support appropriate to their needs throughout the cancer journey.
Information needs
- CUP patients are more likely to want written information about their type of cancer and tests received but are less likely to understand explanations of their condition (Wagland et al. 2017).
- Provide appropriate information to patients and carers about how to manage alterations in cognitive function and potential changes in behaviour.
- Patients may need advice about safe driving.
- Patients from culturally and linguistically diverse backgrounds may need information provided in other formats.
The lead clinician should:
- discuss the treatment plan with the patient and carer, including the intent of treatment and expected outcomes (provide a written plan after final histopathological diagnosis (not frozen section) is available)
- provide the patient and carer with information on possible side effects of treatment, self- management strategies and emergency contacts
- initiate a discussion regarding advance care planning with the patient and carer.
The lead clinician should:
- communicate with the person’s GP about their role in symptom management, psychosocial care and referral to local services
- ensure regular and timely two-way communication with the GP regarding:
- the treatment plan, including intent and potential side effects
- supportive and palliative care requirements
- the patient’s prognosis and their understanding of this
- enrolment in research and/or clinical trials
- changes in treatment or medications
- recommendations from the MDT.
The transition from active treatment to post-treatment care is critical to long-term health. After completing their initial treatment, patients should be provided with a treatment summary and follow- up care plan including a comprehensive list of issues identified by all members of the MDT. Transition from acute to primary or community care will vary depending on the type and stage of cancer and needs to be planned. In some cases, people will require ongoing, hospital-based care.
Survival from CUP is improving over time (Riihimaki et al. 2013b). Between 2010 and 2014, people diagnosed with CUP had a 14 per cent chance of surviving for five years compared with the age- and sex-matched general Australian population. This has increased from 6 per cent between 1984 and 1988 (AIHW 2018). While long-term survival may only apply to a subset of patients, the survivorship needs of all patients need to be considered.
International research shows there is an important need to focus on helping cancer survivors cope with life beyond their acute treatment. Cancer survivors experience particular issues, often different from people having active treatment for cancer. Ongoing support and symptom management should be provided to patients with CUP throughout their care. Empathetic discussion about the prognosis of CUP, along with support and counselling by the treatment team and specialised services, may help alleviate distress (NCCN 2017). Many cancer survivors experience persisting side effects at the end of treatment. Emotional and psychological issues include distress, anxiety, depression, cognitive changes and fear of cancer recurrence. Late effects may occur months or years later and are dependent on the type of cancer treatment. Survivors may experience altered relationships and may encounter practical issues, including difficulties with return to work or study, and financial hardship.
Patients may be discharged into the community and generally need to see a specialist for regular followup appointments. In its report, From cancer patient to cancer survivor: Lost in transition, the former Institute of Medicine (now the National Academy of Medicine) describes four essential components of survivorship care (Hewitt et al. 2006):
- the prevention of recurrent and new cancers, as well as late effects
- surveillance for cancer spread, recurrence or second cancers, and screening and assessment for medical and psychosocial late effects
- interventions to deal with the consequences of cancer and cancer treatments (including management of symptoms, distress and practical issues)
- coordination of care between all providers to ensure the patient’s needs are met.
All patients should be educated in managing their own health needs (NCSI 2015).
After initial treatment, the patient, the patient’s nominated carer (as appropriate) and GP should receive a treatment summary outlining:
- the diagnostic tests performed and results
- tumour characteristics
- the type and date of treatment(s)
- interventions and treatment plans from other health professionals
- supportive care services provided
- contact information for key care providers.
There is no evidence that follow-up investigations of asymptomatic patients with non-specific CUP affects outcome. Patients in the specific-CUP subgroup should be followed up as per disease-specific guidelines. Specific examinations should be undertaken as clinically indicated (Fizazi et al. 2015).
Care in the post-treatment phase is driven by predicted risks (such as the risk of recurrence, developing late effects and psychological issues), as well as individual clinical and supportive care needs.
The responsibility for follow-up care should be agreed between the lead clinician, the person’s GP, relevant members of the MDT and the patient, with an agreed plan that outlines:
- what medical follow-up is required (surveillance for recurrence, screening and assessment for medical and psychosocial effects)
- care plans from other health professionals to manage the consequences of cancer and treatment
- a process for rapid re-entry to specialist medical services for suspected recurrence
- the role of follow-up for patients, which is to evaluate tumour control, monitor and manage symptoms from the tumour and treatment and provide psychological support
- that they will be retained within the MDT management framework
- the arrangements for follow-up with the neurosurgeon for a postoperative evaluation, which should occur four to eight weeks after surgery.
In particular circumstances, follow-up care can safely and effectively be provided:
- in the primary care setting
- by other suitably trained staff (for example, nurse-led follow-up)
- in a non-face-to-face setting (for example, by telehealth).
Screening using a validated screening tool and referral to appropriate health professionals and community-based support services is required to meet the needs of individual patients, their families and carers.
In addition to the other common issues outlined in the Appendix, specific needs that may arise at this time include the following.
Physical needs
- Fatigue/change in functional abilities is a common symptom, and patients may benefit from referral to occupational therapy.
- Decline in mobility and/or functional status may result from treatment.
- Assistance with managing complex medication regimens, multiple medications, assessment of side effects and assistance with difficulties swallowing medications may be required. Refer to a pharmacist if necessary.
Psychological needs
- Patients may need support with emotional and psychological issues including, but not limited to, body image concerns, fatigue, existential anxiety, treatment phobias, anxiety/depression, interpersonal problems and sexuality concerns.
- Many patients with CUP find the uncertainty surrounding their disease and the limited treatment options difficult and would welcome the opportunity to ask questions and learn about others’ experiences (Boyland & Davis 2008).
- Many patients with CUP and their clinicians have a poor understanding of their illness, have difficulty in explaining their illness to others, and have a sense of frustration in health professionals not having the answers (Boyland & Davis 2008, Karapetis et al. 2017).
- Depressive symptoms are higher in people with CUP when compared with people with cancer of a known origin, so they require more psychosocial support and specific interventions (Hyphantis et al. 2013).
- GPs play an important role in coordinating care for patients, including assisting with side effects and offering support when questions or worries arise. For most patients, simultaneous care provided by their GP is very important (Lang et al. 2017).
Social/practical needs
- Patients may need support to attend appointments.
- Potential isolation from normal support networks, particularly for rural patients who are staying away from home for treatment and for patients with neuropsychiatric symptoms, can be an issue. Social isolation can also compound distress (Australian Cancer Network 2009).
- Financial issues related to loss of income and additional expenses as a result of illness and/or treatment may require support.
- Help with legal issues may be required including advance care planning, appointing a power of attorney and completing a will.
Spiritual needs
- Multidisciplinary teams should have access to suitably qualified, authorised and appointed spiritual caregivers who can act as a resource for patients, carers and staff.
- Patients with cancer and their families should have access to spiritual support appropriate to their needs throughout the cancer journey.
Information needs
- CUP patients are more likely to want written information about their type of cancer and tests received but less likely to understand explanations of their condition (Wagland et al. 2017).
- Patients and carers may need appropriate information about how to manage alterations in cognitive function and potential changes in behaviour.
- Patients may need advice about safe driving.
- Patients from culturally and linguistically diverse backgrounds may need information provided in other formats.
Rehabilitation may be required at any point of the care pathway from preparing for treatment through to disease-free survival and palliative care.
Issues that may need to be addressed include managing fatigue, falls, cognitive changes, improving physical endurance, achieving independence in daily tasks, returning to work and ongoing adjustment to disease and its sequelae.
Many people with CUP receive palliative treatment to relieve symptoms and improve quality of life.
In all patients with CUP, palliative care interventions should be considered and utilised as appropriate (NCCN 2017). It is preferable for specialist palliative care to be initiated during the diagnostic stage, and for the majority of patients this will remain the most important intervention during their illness (Abdallah et al. 2014). Palliative care is integral throughout the patient pathway, and people who have undergone early integrated palliative care build rapport with their oncologist and are more likely to discuss end-of-life care wishes (Temel et al. 2017).
Early referral to palliative care can improve the quality of life for people with cancer (Haines 2011, Temel et al. 2010, Temel et al. 2017, Zimmermann et al. 2014) including better management of physical and psychological symptoms. This is particularly true for poor-prognosis cancers (Cancer Council Australia 2017, Philip et al. 2013, Temel et al. 2010, Temel et al. 2017). Furthermore, palliative care has been associated with the improved wellbeing of carers (Higginson 2010, Hudson et al. 2014).
Ensure carers and families receive information, support and guidance regarding their role according to their needs and wishes (Palliative Care Australia 2005).
The patient and carer should be encouraged to develop an advance care plan (AHMAC 2011).
Further information
Refer patients and carers to Palliative Care Australia.
The lead clinician should:
- explain the treatment summary and follow-up care plan
- provide information on the signs and symptoms of recurrent disease
- provide information on secondary prevention and healthy living.
The lead clinician should ensure regular, timely, two-way communication with the patient’s GP regarding:
- the follow-up care plan
- potential late effects
- supportive and palliative care requirements
- the patient’s progress
- recommendations from the MDT
- any shared care arrangements
- a process for rapid re-entry to medical services for patients with suspected recurrence.
Step 6 is concerned with managing recurrent or progressive disease.
The pathway and management of patients with recurrent or progressive CUP is a continuum of care and recapitulates Step 4.
With the low rates of curable CUP and the majority of patients palliated, it is likely that their current symptoms will worsen progressively, and this should be managed following discussion with palliative care specialists.
The supportive care needs of these patients are particularly important and should be reassessed. Palliative care referral and increased support within the community and GP involvement are also particularly important (Temel et al. 2017).
End-of-life care is appropriate when the patient’s symptoms are increasing and functional status is declining. Step 7 is concerned with maintaining the patient’s quality of life and addressing their health and supportive care needs as they approach the end of life, as well as the needs of their family and carer(s). Consideration of appropriate venues of care is essential. The principles of a palliative approach to care need to be shared by the team when making decisions with the patient and their family.
If not already underway, referral to palliative care should be considered at this stage (including nursing, pastoral care, palliative medicine specialist backup, inpatient palliative bed access as required, social work, neuro-psychology/psychiatry and bereavement counselling), with GP engagement.
If not already in place, the patient and carer should be encouraged to develop an advance care plan (AHMAC 2011).
The palliative care team may consider seeking additional expertise from a:
- pain service
- pastoral carer or spiritual advisor
- bereavement counsellor
- therapist (for example, music or art).
The team might also recommend accessing:
- home- and community-based care
- specialist community palliative care workers
- community nursing.
Consideration of an appropriate place of care and preferred place of death is essential.
Ensure carers and families receive information, support and guidance regarding their role according to their needs and wishes (Palliative Care Australia 2005).
Further information
Refer patients and carers to Palliative Care Australia.
Participation in research and clinical trials should be encouraged where available and appropriate.
- Australian Cancer Trials is a national clinical trials database. It provides information on the latest clinical trials in cancer care, including trials that are recruiting new participants. For more information visit the Australian Cancer Trials website.
Screening, assessment and referral to appropriate health professionals is required to meet the identified needs of an individual, their carer(s) and family.
In addition to the other common issues outlined in the Appendix, specific needs that may arise at this time include the following.
Physical needs
- Fatigue/change in functional abilities is a common symptom, and patients may benefit from referral to occupational therapy.
- Decline in mobility and/or functional status may result from treatment.
- Assistance with managing complex medication regimens, multiple medications, assessment of side effects and assistance with difficulties swallowing medications may be required. Refer to a pharmacist if necessary.
Psychological needs
- Patients may need support with emotional and psychological issues including, but not limited to, body image concerns, fatigue, existential anxiety, treatment phobias, anxiety/depression, interpersonal problems and sexuality concerns.
- Many patients with CUP find the uncertainty surrounding their disease and the limited treatment options difficult and would welcome the opportunity to ask questions and learn about others’ experiences (Boyland & Davis 2008).
- Many patients with CUP and their clinicians have a poor understanding of their illness, have difficulty in explaining their illness to others, and have a sense of frustration in health professionals not having the answers (Boyland & Davis 2008, Karapetis et al. 2016).
- Depressive symptoms are higher in people with CUP when compared with people with cancer of a known origin, so they require more psychosocial support and specific interventions (Hyphantis et al. 2013).
- GPs play an important role in coordinating care for patients, including assisting with side effects and offering support when questions or worries arise. For most patients, simultaneous care provided by their GP is very important (Lang et al. 2017).
Social/practical needs
- Ensure the patient attends appointments.
- Potential isolation from normal support networks, particularly for rural patients who are staying away from home for treatment and for patients with neuropsychiatric symptoms, can be an issue. Social isolation can also compound distress (Australian Cancer Network 2009).
- Financial issues related to loss of income and additional expenses as a result of illness and/or treatment may require support.
- Help with legal issues may be required including advance care planning, appointing a power of attorney and completing a will.
Spiritual needs
- Multidisciplinary teams should have access to suitably qualified, authorised and appointed spiritual caregivers who can act as a resource for patients, carers and staff.
- Provide bereavement support for family and friends.
- Patients with cancer and their families should have access to spiritual support appropriate to their needs throughout the cancer journey.
- Cater to specific spiritual needs that may benefit from the involvement of pastoral care.
Information needs
- CUP patients are more likely to want written information about their type of cancer and tests received but less likely to understand explanations of their condition (Wagland et al. 2017).
- Provide information for patients and families about arranging a funeral.
- Communicate about the death and dying process and what to expect.
- Communicate with all members of the care team that the patient has died.
- Patients from culturally and linguistically diverse backgrounds may need information provided in other formats.
The lead clinician should:
- be open to and encourage discussion about the expected disease course, with due consideration to personal and cultural beliefs and expectations
- discuss palliative care options including inpatient and community-based services as well as dying at home and subsequent arrangements
- provide the patient and carer with the contact details of a palliative care service.
The lead clinician should discuss end-of-life care planning and transition planning to ensure the patient’s needs and goals are addressed in the appropriate environment. The patient’s GP should be kept fully informed and involved in major developments in the patient’s illness trajectory.
Planning and delivering appropriate cancer care for older people presents a number of challenges. Improved communication between the fields of oncology and geriatrics is required to facilitate the delivery of best practice care, which takes into account physiological age, complex comorbidities, risk of adverse events and drug interactions as well as the implications of cognitive impairment on the suitability of treatment and consent (Steer et al. 2009).
A national interdisciplinary workshop convened by the Clinical Oncology Society of Australia (COSA) recommended that people over the age of 70 undergo some form of geriatric assessment in line with international guidelines (COSA 2013). Assessment can be used to determine life expectancy and treatment tolerance as well as identifying conditions that might interfere with treatment including:
- function
- comorbidity
- presence of geriatric syndromes
- nutrition
- polypharmacy
- cognition
- emotional status
- social supports.
Recent years have seen the emergence of adolescent and young adult (AYA) oncology as a distinct field due to lack of progress in survival and quality-of-life outcomes (Ferrari et al. 2010, NCI & USDHHS 2006, Smith et al. 2013). The significant developmental change that occurs during this life stage complicates a diagnosis of cancer during the AYA years, often leading to unique physical, social and emotional impacts for young people at the time of diagnosis and throughout the cancer journey (Smith et al. 2012).
In caring for young people with cancer, careful attention to promoting normal development is required (COSA 2011). This requires personalised assessments and management involving a multidisciplinary, disease-specific, developmentally targeted approach informed by:
- understanding the developmental stages of adolescence and supporting normal adolescent health and development alongside cancer management
- understanding and supporting the rights of young people
- communication skills and information delivery that are appropriate to the young person
- addressing the needs of all involved, including the young person, their family and/or carer(s)
- working with educational institutions and workplaces
- addressing survivorship and palliative care needs.
An oncology team caring for a young person with cancer must:
- ensure access to expert AYA health professionals with knowledge specific to the biomedical and psychosocial needs of the population
- understand the biology and current management of the disease in the AYA age group
- consider clinical trials accessibility and recruitment for each patient
- if the prognosis is favourable, engage in proactive discussions about fertility preservation and the late effects of treatment and consider the patient’s psychosocial needs
- provide treatment in an AYA-friendly environment.
Youth cancer services
Youth cancer services provide specialist, age-appropriate treatment and support for young cancer patients aged 15–25 years. Youth cancer services are provided at five lead hospitals and linked to a network of more than 25 hospitals across Australia. Each service has a multidisciplinary specialist team of doctors and health professionals who are experienced in treating and caring for young people with a range of different cancers. They can also provide advice and information about the specific needs of AYA with a cancer diagnosis.
The burden of cancer is higher in the Australian Indigenous population (AIHW 2014). Survival also significantly decreases as remoteness increases. Aboriginal and Torres Strait Islander people in Australia have high rates of certain lifestyle risk factors including tobacco smoking, higher alcohol consumption, poor diet and low levels of physical activity (Cancer Australia 2015). The high prevalence of these risk factors is believed to be a significant contributing factor to the patterns of cancer incidence and mortality rates in this population group (Robotin et al. 2008).
In caring for Aboriginal and Torres Strait Islander people diagnosed with cancer, the current gap in survivorship is a significant issue. The following approaches are recommended to improve survivorship outcomes (Cancer Australia 2015):
- Raise awareness of risk factors and deliver key cancer messages.
- Develop evidence-based information and resources for community and health professionals.
- Provide training for Aboriginal and Torres Strait Islander health workers and develop training resources.
- Increase understanding of barriers to care and support.
- Encourage and fund research.
- Improve knowledge within the community to act on cancer risk and symptoms.
- Improve the capacity of Aboriginal and Torres Strait Islander health workers to provide cancer care and support to their communities.
- Improve system responsiveness to cultural needs.
- Improve our understanding of care gaps through data monitoring and targeted priority research.
For people from diverse backgrounds in Australia, a cancer diagnosis can come with additional complexities, particularly when English proficiency is poor. In some languages there is not a direct translation of the word ‘cancer’, which can make communicating vital information difficult. Perceptions of cancer and related issues can differ greatly in those from culturally diverse backgrounds and can impact on the understanding and decision making that follows a cancer diagnosis. In addition to different cultural beliefs, when English language skills are limited there is potential for miscommunication of important information and advice, which can lead to increased stress and anxiety for patients. A professionally trained interpreter (not a family member or friend) should be made available when communicating with people with limited English proficiency.
Navigation of the Australian healthcare system can pose problems for those born overseas, and particular attention should be paid to supporting these patients (Department of Health 2009).
Supportive care in cancer refers to the following five domains:
- physical domain, which includes a wide range of physical symptoms that may be acute, relatively short-lived or ongoing, requiring continuing interventions or rehabilitation (NBCC & NCCI 2003)
- psychological domain, which includes a range of issues related to the person’s mental health and personal relationships (NBCC & NCCI 2003)
- social domain, which includes a range of social and practical issues that will impact on the individual and family such as the need for emotional support, maintaining social networks and financial concerns (NICE 2004)
- information domain, which includes access to information about cancer and its treatment, support services and the health system overall (NBCC & NCCI 2003)
- spiritual domain, which focuses on the person’s changing sense of self and challenges to their underlying beliefs and existential concerns (NICE 2004).
Fitch’s (2000) model of supportive care recognises the variety and level of intervention required at each critical point as well as the need to be specific to the individual. The model targets the type and level of intervention required to meet patients’ supportive care needs.
Some patients with CUP will require specialised intervention. Common indicators in patients with CUP that may require referral to appropriate health professionals and/or organisations include the following.
Physical needs
- Patients should receive treatment for physical symptoms.
- Breathlessness and coughing often create anxiety. Consider strategies such as exploring the meaning of breathlessness, breathing retention exercises, teaching relaxation and distraction techniques, and encouraging planning and pacing (NBCC & NCCI 2003). Referral to physiotherapy, occupational therapy or pulmonary rehabilitation may be required.
- If oxygen is medically indicated, this can be arranged through the relevant state aids and equipment program.
- Alteration of cognitive functioning in patients treated with chemotherapy and radiation therapy requires strategies such as maintaining written notes or a diary and repetition of information (NBCC & NCCI 2003).
- Patients with weight loss or bony metastasis often experience pain sitting or lying. Appropriate devices should be considered to improve overall comfort and engagement in activities.
- Physical decline is common in patients with CUP. Maintenance of function should be encouraged. Referral to a physiotherapist should be considered if further advice is required.
- Should a patient’s physical function deteriorate, referral to occupational therapy for review of their home environment should be considered if compensation strategies are required.
- Referral to a pharmacist may be useful for people managing multiple medications.
- Decline in mobility and/or functional status as a result of treatment should be monitored.
- Malnutrition can occur as a result of disease or treatment. Validated malnutrition screening tools should be used at the key points in the care pathway to identify patients at risk of malnutrition and refer to a dietitian for nutrition intervention.
- Fatigue/change in functional capacity is a common symptom, and patients may benefit from referral to occupational therapy.
- Focal deficits may affect the patient’s mobility and ability to take part in everyday activities. Referral to an occupational therapist and a physiotherapist for assessment, education, intervention and compensatory strategies may assist with maintaining mobility.
- Dysphasia may occur, and referral to a speech pathologist may be needed (Taillibert et al. 2004).
Psychological needs
- Many patients with CUP find the uncertainty surrounding their disease and the inability to treat it difficult and would welcome the opportunity to ask questions and learn about others’ experiences (Boyland & Davis 2008).
- Many patients with CUP and their clinicians have a poor understanding of their illness, have difficulty in explaining their illness to others, and have a sense of frustration in health professionals not having the answers (Boyland & Davis 2008, Karapetis et al. 2016).
- It is important to ensure the patient understands that they are being investigated for a possible cancer diagnosis, even prior to a tissue diagnosis being obtained.
- Patients with CUP will most likely undergo extensive testing, and support and information should be provided to assist with any distress while undergoing these tests (Wagland et al. 2017).
- The GP should inform the patient that they might have cancer early on in the diagnostic investigation process. Good communication from experts at this stage is very important.
- Depressive symptoms are higher in people with CUP when compared with people with cancer of a known origin, so they require more psychosocial support and specific interventions (Hyphantis et al. 2013).
- Palliative care can improve physical symptom control and quality of life for people with cancer including improving the capacity of patients to come to terms with diagnostic and prognostic uncertainty (Temel et al. 2017).
- GPs play an important role in coordinating care for patients, including assisting with side effects and offering support when questions or worries arise. For most patients, simultaneous care provided by their GP is very important (Lang et al. 2017).
- For some populations (culturally and linguistically diverse backgrounds, Aboriginal and Torres Strait Islanders, and lesbian, gay, bisexual, transgender and intersex (LGBTI) communities) a cancer diagnosis can come with additional psycho-social complexities. Access to expert health
- professionals with knowledge specific to the psychosocial needs of these groups may be required.
- Patients with CUP should be regularly screened for depression and anxiety as a result of increased dependency. If loss of independence is a factor contributing to depression, then referral to physiotherapy and occupational therapy may restore some independence and assist some people. Referral to a psychologist or psychiatrist may also be helpful in managing the depression.
- Some people may have disabling symptoms and may benefit from referral to a psychology service.
- Distress and depression can be just as common in carers and family members, including children.
- Cognitive changes as a result of treatment (such as altered memory, attention and concentration) may require assessment.
- Help with the emotional distress of dealing with a cancer diagnosis, stress and adjustment difficulties may be required (Hyphantis et al. 2015).
- Difficulty with social interactions can place the patient at higher risk of depression. A referral to a speech pathologist to manage and maximise any communication impairments, and an occupational therapist or psychologist for social skills training, may help to reduce psychosocial difficulties.
- Patients may need support with body image concerns, fatigue, existential anxiety, treatment phobias, anxiety/depression, interpersonal problems, returning to work, and sexuality concerns.
- Consider a referral to a psychologist, psychiatrist or social worker if the patient is:
- displaying emotional cues such as tearfulness, distress, avoidance and withdrawal
- preoccupied with or dwelling on thoughts about cancer and death
- displaying fears about the treatment process and/or the changed goals of their treatment
- worried about loss associated with their daily function, dependence on others and loss of dignity
- becoming isolated from family and friends and withdrawing from people and activities that they previously enjoyed
- feeling hopeless and helpless about the impact that brain cancer is having on their life and the disruption to their life plans
- struggling with communicating to family and loved ones about the implications of their cancer diagnosis and treatment
- experiencing changes in sexual intimacy, libido and function
- struggling with the diagnosis of metastatic or advanced disease
- having difficulties transitioning to palliative care.
Social/practical needs
- A diagnosis of CUP can have significant financial, social and practical impacts on patients, carers and families.
- The additional costs related to management are significant. A referral to a social worker should be considered for further assessment and identification of financial and practical support available.
- Significant restrictions to social activities may require referral to a social worker, occupational therapist, psychologist or psychiatrist.
- Patients may need advice about safe driving.
- Consider the need for appropriate information for people from culturally and linguistically diverse backgrounds.
Information needs
- Patients with CUP will most likely undergo extensive testing, and support and information should be provided to assist with any distress while undergoing these tests (Wagland et al. 2017).
- CUP patients are more likely to want written information about their type of cancer and tests received but less likely to understand explanations of their condition (Wagland et al. 2017).
- Ensure the patient understands that they have been diagnosed with CUP, and that they understand the ramifications of this diagnosis.
- Assist the patient in explaining their diagnosis to their family. Audio recordings may be a helpful resource for patients (Pitkethly et al. 2008).
- Offer individualised cancer information that meets the needs of the patient and carer (this may involve advice from health professionals as well as written and visual resources).
- Offer advice on how to access information from websites, community sources and national cancer services. A list of websites has been included under Resource List.
Spiritual needs
- Multidisciplinary teams should have access to suitably qualified, authorised and appointed spiritual caregivers who can act as a resource for patients, carers and staff. They should also have up-to date awareness of local community resources for spiritual care.
- Patients with cancer and their families should have access to spiritual support appropriate to their needs throughout the cancer journey.
Australian Cancer Survivorship Centre
Has general and tumour-specific information, primarily focused on the post-treatment survivorship phase
- Telephone: (03) 9656 5207
- Website
Beyond Blue
Information on depression, anxiety and related disorders, available treatment and support services
- Telephone: 1300 22 4636
- Website
Cancer Australia
Information on cancer prevention, screening, diagnosis, treatment and supportive care for Australians affected by cancer, and their families and carers
- Telephone: 1800 624 973
- Website
Cancer Council Australia
A confidential telephone support service for people affected by cancer that provides
information on treatment, cancer support groups and other community resources
- Telephone: 13 11 20 (Monday to Friday, 9.00 am – 5.00 pm)
- Website
- Understanding cancer of unknown primary – a guide for people with cancer, their families and friends
Cancer of Unknown Primary Foundation – Jo’s friends
A UK-based website for people who have been touched by CUP. Contains information on cancer diagnosis, treatment, supportive care and research
CanTeen
Australian organisation for young people living with cancer that offers support, information and resources. CanTeen is available for people aged 12–25 who are affected by cancer, such as those diagnosed, their siblings or the children of parents or carers with a cancer diagnosis
- Telephone: 1800 226 833
- Website
Care Search: Palliative Care Knowledge Network
Information for patients and carers on living with illness, practical advice on how to care, and finding services
- Telephone: (08) 7221 8233
- Website
Look Good, Feel Better
A non-medical, free community service program dedicated to teaching women how to manage the appearance-related side effects caused by cancer treatment
- Telephone: 1800 650 960
(Monday to Thursday, 9 am – 5 pm)
Australian Cancer Trials
Information on the latest clinical trials in cancer care, including trials that are recruiting new participants
Australasian Lymphology Association
Professional organisation promoting best practice in lymphedema management, research and education. Provides a public register of lymphoedema practitioners in Australia and New Zealand
Cancer Australia
Information for health professionals including guidelines, cancer guides, reports, fact sheets, DVDs, posters and pamphlets
Cancer Council Australia
Information on prevention, research, treatment and support provided by Australia’s peak independent cancer authority
CareSearch
Information about palliative care for patients, carers and families as well as for the health professionals providing their care
Clinical trials app
The TROG Cancer Research app provides information about cancer research trials for patients and clinicians
- Download the TROG Cancer Research app
ESMO guidelines
Clinical practice guidelines on cancer of unknown primary
NCCN guidelines
Clinical practice guidelines on cancer of unknown primary
National Health and Medical Research Council
Information on clinical practice guidelines, cancer prevention and treatment
Rare Cancers Australia
Information on rare and less common cancers in Australia
Youth cancer services
Youth cancer services provide specialist, age- appropriate treatment and support for young cancer patients aged 15–25 years
Advance care directive – voluntary person-led document that focus on an individual’s values and preferences for future health and medical treatment decisions, preferred outcomes and care. They are completed and signed by a competent person. They are recognised by specific legislation (statutory) or common law (non-statutory). Advance care directives can also appoint the substitute decision-maker(s) who can make decisions about health or personal care on the individual’s behalf if they are no longer able to make decisions themselves. Advance care directives focus on the future health care of a person, not on the management of his or her assets. They come into effect when an individual loses decision-making capacity.
Advance care planning – the process of planning for future health and personal care, where the person’s values, beliefs and preferences are made known so they can guide decision making at a future time when that person cannot make or communicate their decisions.
Alternative therapies – treatments used in place of conventional medical treatment.
Care coordinator – the health provider nominated by the multidisciplinary team to coordinate patient care. The care coordinator may change over time depending on the patient’s stage in the care pathway and the location and care in which care is being delivered.
Castrate resistant progressive disease – progressive disease despite castrate levels of testosterone (Cancer Council Australia Advanced Prostate Cancer Guidelines Working Party 2010).
Complementary therapies – supportive treatment used in conjunction with conventional medical treatment. These treatments may improve wellbeing and quality of life and help people deal with the side effects of cancer.
End-of-life care – includes physical, spiritual and psychosocial assessment, and care and treatment, delivered by health professionals and ancillary staff. It also includes support of families and carers and care of the patient’s body after their death.
Immunotherapy – a type of cancer treatment that helps the body’s immune system to fight cancer. Immunotherapy can boost the immune system to work better against cancer or remove barriers to the immune system attacking the cancer.
Indicator – a documentable or measurable piece of information regarding a recommendation in the optimal care pathway.
Informed financial consent – the provision of cost information to patients, including notification of likely out-of-pocket expenses (gaps), by all relevant service providers, preferably in writing, before admission to hospital or treatment (Commonwealth Department of Health 2017).
Lead clinician – the clinician who is nominated as being responsible for individual patient care. The lead clinician may change over time depending on the stage of the care pathway and where care is being provided.
Metastatic disease – cancer that has spread from the part of the body where it started (the primary site) to other parts of the body.
Multidisciplinary care – an integrated team approach to health care in which medical and allied health providers consider all relevant treatment options and collaboratively develop an individual treatment plan for each patient.
Multidisciplinary team – comprises the core disciplines that are integral to providing good care. The team is flexible in approach, reflects the patient’s clinical and psychosocial needs and has processes to facilitate good communication.
Multidisciplinary team meeting – a meeting of health professionals from one or more clinical disciplines who together make decisions about recommended treatment of patients.
Optimal care pathway – the key principles and practices required at each stage of the care pathway to guide the delivery of consistent, safe, high-quality and evidence-based care for all people affected by cancer.
Palliative care – any form of medical care or treatment that concentrates on reducing the severity of disease symptoms.
Patient management frameworks – tumour stream models adopted in Victoria in 2003 to reduce variation in cancer care. The optimal care pathways are updated versions of these models.
Performance status – an objective measure of how well a patient can carry out activities of daily life.
Prehabilitation – one or more interventions performed in a newly diagnosed cancer patient that are designed to improve physical and mental health outcomes as the patient undergoes treatment and beyond.
Primary care health professional – in most cases this is a general practitioner but may also include general practice nurses, community nurses, nurse practitioners, allied health professionals, midwives, pharmacists, dentists and Aboriginal health workers.
Primary specialist – the person who makes the referral to the multidisciplinary team (such as specialist physician, surgeon, oncologist, palliative care specialist). This person will also make referrals for treatment and will be responsible for overseeing follow-up care.
PSMA PET/CT – a whole body scan that images prostate cancer wherever it is located in the body.
Rehabilitation – comprises multidisciplinary efforts to allow the patient to achieve optimal physical, social, physiological and vocational functioning within the limits imposed by the disease and its treatment.
Spiritual care – the aspect of humanity that refers to the way individuals seek and express meaning and purpose and the way they experience their connectedness to the moment, to self, to others, to nature, and to the significant or sacred.
Substitute decision-maker – a person permitted under the law to make decisions on behalf of someone who does not have competence or capacity.
Supportive care – care and support that aims to improve the quality of life of people living with cancer, cancer survivors and their family and carers and particular forms of care that supplement clinical treatment modalities.
Survivorship – an individual is considered a cancer survivor from the time of diagnosis, and throughout their life; the term includes individuals receiving initial or maintenance treatment, in recovery or in the post-treatment phase.
Survivorship care plan – a formal, written document that provides details of a person’s cancer diagnosis and treatment, potential late and long-term effects arising from the cancer and its treatment, recommended follow-up, surveillance, and strategies to remain well.
Targeted therapy – a medicine that blocks the growth and spread of cancer by interfering with specific molecules.
We acknowledge the Traditional Owners of Country throughout Australia and their continuing connection to the land, sea and community. We pay our respects to them and their cultures and to Elders past, present and emerging.
This work is available from the Cancer Council website.
First published in January 2020.
ISBN: 978 1 76096 154 1
Cancer Council Victoria and Department of Health Victoria 2021, Optimal care pathway for people with cancer of unknown primary, Cancer Council Victoria, Melbourne.
Enquiries about this publication can be sent to optimalcare.pathways@cancervic.org.au.
Our thanks to the following health professionals, consumer representatives, stakeholders and organisations consulted in the development of this optimal care pathway.
Assoc. Prof. Linda Mileshkin (Chair), Medical Oncologist, Peter MacCallum Cancer Centre Dr Amey Aurangabadkar, Radiologist, Illawarra Radiology Group
Prof. David Ball, Radiation Oncologist, Peter MacCallum Cancer Centre
Prof. David Bowtell, Genomics Specialist, Peter MacCallum Cancer Centre
Ms Cindy Bryant, Consumer representative and nurse
Ms Julie Callaghan, System Improvement Program Lead, Cancer Institute NSW
Dr Tina Chen, Medical and Scientific Advisor, Cancer Institute NSW
Prof. Jonathan Clark, Head and Neck Surgeon, Chris O’Brien Lifehouse
Prof. Katherine Clark, Palliative Care Physician, North Sydney Local Health District
Prof. David Currow, Chief Cancer Officer NSW and Chief Executive Officer Cancer Institute NSW Prof. Anthony Gill, Pathologist, Sydney University Dr Julie Gottlieb, General Practitioner and Senior Medical Adviser at the Health Care Complaints Commission
Ms Laura Holliday, Project Officer Medical and Scientific Advice Team, Cancer Institute NSW
Dr Laura Kirsten, Clinical Psychologist, NBMLHD Psychology, Nepean Cancer Care Centre
Ms Nina Klug, Project Officer System Improvement, Cancer Institute NSW Assoc. Prof. Sally Lord, Epidemiologist, The University of Notre Dame, Australia Dr Catherine Mason, Psychiatrist, Crown Princess Mary Cancer Centre,
Nepean Cancer Care Centre, University of Sydney Dr Siobhan O’Neill, Medical Oncologist,
Prince of Wales Hospital
Dr Richard Osborne, Medical Oncologist, Hervey Bay Hospital
Prof. Penny Schofield, Behavioural Scientist, Peter MacCallum Cancer Centre
Prof. Martin Tattersall, Medical Oncologist, The University of Sydney
Prof. Robert (Bob) Thomas, Surgeon, The University of Melbourne
Dr Richard Tothill, Genomics Specialist, Peter MacCallum Cancer Centre
Assoc. Prof. Claire Vajdic, Epidemiologist, UNSW Sydney
Prof. Desmond Yip, Medical Oncologist, The Canberra Hospital and Australian National University
Governance – project steering committee representation
Cancer Australia Cancer Council Victoria
Cancer Institute New South Wales Consumer representatives
Department of Health and Human Services, Cancer Strategy and Development
Monash University
North Eastern Melbourne Integrated Cancer Service
Olivia Newton-John Cancer Wellness & Research Centre
Peter MacCallum Cancer Centre The University of Melbourne Wyong Hospital
Allied Health Professions Australia
Australian Society of Gynaecological Oncologists Australian Association of
Nuclear Medicine Specialists Australian and New Zealand Gynaecological Oncology Group Australian and New Zealand Society of Palliative Care
Australian Chapter of Palliative Medicine, Royal Australasian College of Physicians Australian College of Nursing
Australian Institute of Radiography Australian Medical Association
Interventional Radiology Society of Australasia Medical Oncology Group of Australia
Royal Australasian College of Physicians Royal Australasian College of Surgeons Royal Australian and New Zealand College of Psychiatrists
Royal Australian and New Zealand College of Radiologists
Royal Australian College of General Practitioners
Other stakeholders consulted to provide feedback including Cancer Action Victoria, a number of health services and integrated cancer services.
Abdallah S, Agbamu D, Aurangabadkar A, Carser J, Helliwell T, Husband D, et al. 2014, Guidelines for the investigation and management of metastatic malignant disease of unknown primary origin (V 1.2). NHS Cheshire & Merseyside Strategic Clinical Networks.
Australian Cancer Network Adult Brain Tumour Guidelines Working Party 2009, Clinical practice guidelines for the management of adult gliomas: astrocytomas and oligodendrogliomas. Cancer Council Australia, Australian Cancer Network and Clinical Oncological Society of Australia Inc., Sydney.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2004, Standard for credentialing and defining the scope of clinical practice, ACSQHC, Sydney.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2011, National Safety and Quality Health Service Standards, ACSQHC, Sydney, viewed March 2015, <http://www.safetyandquality.gov.au/wp- content/uploads/2011/09/NSQHS-Standards- Sept-2012.pdf>.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2013, Consumers, the health system and health literacy: taking action to improve safety and quality, Consultation Paper, ACSQHC, Sydney.
Australian Health Ministers’ Advisory Council (AHMAC) 2011, A national framework for advance care directives, AHMAC, Canberra, viewed November 2017, <http://www.coaghealthcouncil.gov.au/ Portals/0/A%20National%20Framework%20 for%20Advance%20Care%20Directives_ September%202011.pdf>.
Australian Institute of Health and Welfare (AIHW) 2014, Cancer in Australia: an overview 2014, Cancer series No. 90. Cat. no. CAN 88, AIHW, Canberra.
Australian Institute of Health and Welfare (AIHW) 2018, Cancer compendium: information and trends by cancer type; Cancer of unknown primary site in Australia, AIHW, Canberra.
Berner A, Davidson B, Sigstad E, Risberg B 2003, ‘Fine-needle aspiration cytology vs core biopsy in the diagnosis of breast lesions.’ Diagn Cytopathol 29(6): 344–348.
Boyland L, Davis C 2008, ‘Patients’ experiences of carcinoma of unknown primary site: dealing with uncertainty.’ Palliat Med 22(2): 177–183.
Brandes K, Linn AJ, Butow PN, van Weert JC 2014, ‘The characteristics and effectiveness of Question Prompt List interventions
in oncology: a systematic review of the literature.’ Psychooncology, viewed September 2014, <http://www.ncbi.nlm.nih.gov/ pubmed/25082386>.
Brewster DH, Lang J, Bhatti LA, Thomson CS, Oien KA 2014, ‘Descriptive epidemiology of cancer of unknown primary site in Scotland, 1961–2010.’ Cancer Epidemiol 38(3): 227–234.
Burglin SA, Hess S, Høilund-Carlsen PF, Gerke O 2017, ‘18F-FDG PET/CT for detection of the primary tumor in adults with extracervical metastases from cancer of unknown primary: a systematic review and meta-analysis.’ Medicine (Baltimore) 96(16): e6713.
Cancer Australia 2010, Complementary and alternative therapies, Cancer Australia, Surry Hills, NSW, viewed October 2013, <https:// canceraustralia.gov.au/publications-and- resources/position-statements/complementary- and-alternative-therapies>.
Cancer Australia 2015, National Aboriginal and Torres Strait Islander cancer framework, Cancer Australia, Surry Hills, NSW, viewed April 2018, <https://canceraustralia.gov.au/sites/default/ files/publications/national-aboriginal-and-torres- strait-islander-cancer-framework/pdf/2015_atsi_ framework_1.pdf>.
Cancer Council Australia 2017, Cancer of unknown primary, viewed October 2017,
<http://www.cancer.org.au/about-cancer/types- of-cancer/cancer-of-unknown-primary.html>.
Clinical Oncology Society of Australia (COSA) 2011, Psychosocial management of AYAs diagnosed with cancer: guidance for health professionals, COSA, Sydney, viewed January 2012, <http://wiki.cancer.org.au/australia/COSA:Psychosocial_management_of_AYA_ cancer_patients>.
Clinical Oncology Society of Australia (COSA) 2013, Annual Scientific Meeting and Workshop.
Culine S, Lortholary A, Voigt JJ, Bugat R, Théodore C, Priou F, et al.; Trial for the French Study Group on Carcinomas of Unknown Primary (GEFCAPI 01) 2003, ‘Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study – trial for the French Study Group on Carcinomas of Unknown Primary (GEFCAPI 01).’ J Clin Oncol 21(18): 3479–3482.
Department of Health 2007a, Achieving best practice cancer care: a guide for implementing multidisciplinary care, State Government of Victoria, Melbourne.
Department of Health 2007b, Linking cancer care: a guide for implementing coordinated cancer care, State Government of Victoria, Melbourne.
Department of Health 2009, Cultural responsiveness framework: Guidelines for Victorian health service.
Ferrari A, Thomas D, Franklin A, Hayes-Lattin B , Mascarin M, van der Graaf W, et al. 2010, ‘Starting an adolescent and young adult program: some success stories and some obstacles to overcome.’ J Clin Oncol, 28(32): 4850–4857.
Fitch M 2000, ‘Supportive care for cancer patients.’ Hosp Q 3(4): 39–46.
Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G 2015, ‘Cancers of unknown primary site: ESMO clinical practice guidelines for diagnosis, treatment and follow- up.’ Ann Oncol 26 (suppl_5): v133–v138.
Gatalica Z, Millis SZ, Vranic S, Bender R, Basu GD, Voss A, et al. 2014, ‘Comprehensive tumor profiling identifies numerous biomarkers of drug response in cancers of unknown primary site: analysis of 1806 cases.’ Oncotarget 5(23): 12440–12447.
Greco A 2013, ‘Cancer of unknown primary site: improved patient management with molecular and immunohistochemical diagnosis.’ ASCO Meeting Library, viewed October 2017,
<https://meetinglibrary.asco.org/record/78842/ edbook#fulltext>.
Gröschel S, Bommer M, Hutter B, Budczies J, Bonekamp D, Heining C, et al. 2016, ‘Integration of genomics and histology revises diagnosis and enables effective therapy of refractory cancer of unknown primary with PDL1 amplification.’ Cold Spring Harb Mol Case Stud 2(6): a001180.
Gross-Goupil M, Fourcade A, Blot E, Penel N, Négrier S, Culine S, et al. 2012, ‘Cisplatin alone or combined with gemcitabine in carcinomas of unknown primary: results of the randomised GEFCAPI 02 trial.’ Eur J Cancer 48(5): 721–727.
Haines IE 2011, ‘Managing patients with advanced cancer: the benefits of early referral for palliative care.’ Med J Aust 194(3): 107–108.
Hainsworth JD, Spigel DR, Clark BL, Shipley D, Thompson DS, Farley C, et al. 2010, ‘Paclitaxel/ carboplatin/etoposide versus gemcitabine/ irinotecan in the first-line treatment of patients with carcinoma of unknown primary site: a randomized, phase III Sarah Cannon Oncology Research Consortium Trial.’ Cancer J 16(1): 70–75.
Hewitt M, Greenfield S, Stovall E 2006, From cancer patient to cancer survivor: lost in transition, National Academies Press, Washington.
Higginson IJ, Evans CJ 2010, ‘What is the evidence that palliative care teams improve outcomes for cancer patients and their families?’ Cancer J 16(5): 423–435.
Hudson P, Trauer T, Kelly B, O’Connor M, Thomas K, Zordan R, et al. 2014, ‘Reducing the psychological distress of family caregivers of home based palliative care patients: longer term effects from a randomised controlled trial.’ Psycho-Oncology, 24(1): 19–24.
Hyphantis T, Papadimitriou I, Petrakis D, Fountzilas G, Repana D, Assimakopoulos K, et al. 2013, ‘Psychiatric manifestations, personality traits and health-related quality of life in cancer of unknown primary site.’ Psychooncology 22(9): 2009–2015.
Janssen S, Glanzmann C, Huber G, Studer G 2014, ‘Individualized IMRT treatment approach for cervical lymph node metastases of unknown primary.’ Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al]. 190(4): 386–393.
Kaaks R, Sookthai D, Hemminki K, Kramer A, Boeing H, Wirfalt E, et al. 2014, ‘Risk factors for cancers of unknown primary site: results from the prospective EPIC cohort.’ Int J Cancer 135(10): 2475–2481.
Karapetis CS, Guccione L, Tattersall MH, Gooden H, Vajdic CM, Lambert S, et al. 2017, ‘Perceptions of cancer of unknown primary site: a national survey of Australian medical oncologists.’ Intern Med J 47(4): 408–414.
Lang V, Walter S, Fessler J, Koester MJ, Ruetters D, Huebner J 2017, ‘The role of the general practitioner in cancer care: a survey of the patients’ perspective.’ J Cancer Res Clin Oncol 143(5): 895–904.
Losa F, Soler G, Casado A, Estival A, Fernández I, Giménez S, et al. 2018, ‘SEOM clinical guideline on unknown primary cancer.’ Clin Transl Oncol 20(1): 89–96.
Luke C, Koczwara B, Karapetis C, Pittman K, Price T, Kotasek D, et al. 2008, ‘Exploring
the epidemiological characteristics of cancers of unknown primary site in an Australian population: implications for research and clinical care.’ Aust N Z J Public Health 32(4): 383–389.
National Breast Cancer Centre (NBCC), National Cancer Control Initiative (NCCI) 2003, Clinical practice guidelines for the psychosocial care of adults with cancer, NBCC, Camperdown.
National Cancer Institute (NCI), US Department of Health and Human Services (USDHHS) 2006, Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000, National Cancer Institute, National Institutes of Health, Bethesda, MD.
National Cancer Survivorship Initiative (NCSI) 2015, Stratified pathways of care, NHS England.
National Comprehensive Cancer Guidelines (NCCN) 2017, Occult primary (cancer of unknown primary [CUP]), Version 2.2017 – October 17, 2016.
National Institute for Clinical Excellence (NICE) 2004, Guidance on cancer service – improving supportive and palliative care for adults with cancer, NICE, London, UK, viewed October 2013, <http://guidance.nice.org.uk/CSGSP>.
National Institute for Health and Care Excellence (NICE) 2010, Diagnosis and management
of metastatic malignant disease of unknown primary origin, viewed November 2017, <https://www.nice.org.uk/guidance/cg104/evidence/full-guideline-pdf-134697133>.
Palliative Care Australia 2005, Standards for providing quality palliative care for all Australians (4th edition), Palliative Care Australia, Deakin.
Park SY, Kim BH, Kim JH, Lee S, Kang GH 2007, ‘Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma.’ Arch Pathol Lab Med 131(10): 1561–1567.
Pavlidis N, Fizazi K 2005, ‘Cancer of unknown primary (CUP).’ Crit Rev Oncol Hematol 54(3): 243–250.
Pavlidis N, Pentheroudakis G 2012, ‘Cancer of unknown primary site.’ Lancet 379(9824): 1428–1435.
Pavlidis N, Khaled H, Gaafar R 2015, ‘A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists.’ J Adv Res 6(3): 375–382.
Peppercorn J, Weeks J, Cook F, Joffe S 2004, ‘Comparison of outcomes in cancer patients treated within and outside cli0.nical trials: conceptual framework and structured review.’ Lancet 363: 263–270.
Philip J, Collins A, Brand C, Moore G, Lethborg C, Sundararajan V, Murphy M, Gold M, 2013, ‘“I’m just waiting…”: an exploration of the experience of living and dying with primary malignant glioma’, Support Care Cancer, Springer-Verlag Berlin, Melbourne.
Pitkethly M, Macgillivray S, Ryan R 2008, ‘Recordings or summaries of consultations for people with cancer.’ Cochrane Database Syst Rev 2008(3): Cd001539.
Rich SE, Mendenhall WM 2016, ‘Short- course radiation for palliation of squamous cell carcinoma of an unknown primary of the head and neck.’ J Clin Oncol 34(26) (October): 62–62.
Riihimaki M, Thomsen H, Hemminki A, Sundquist K, Hemminki K 2013a, ‘Comparison of survival of patients with metastases from known versus unknown primaries: survival in metastatic cancer.’ BMC Cancer 13: 36.
Riihimaki M, Hemminki A, Sundquist K, Hemminki K 2013b, ‘Time trends in survival from cancer of unknown primary: small steps forward.’ Eur J Cancer 49(10): 2403–2410.
Robotin MC, George J, Supramaniam R, Sitas F, Penman A 2008, ‘Preventing primary liver cancer: how well are we faring towards a national hepatitis B strategy?’ Med J Aust 188(6): 363–365.
Ross JS, Wang K, Gay L, Otto GA, White E, Iwanik K, et al. 2015, ‘Comprehensive genomic profiling of carcinoma of unknown primary site: new routes to targeted therapies.’ JAMA Oncol 1(1): 40–49.
Schaffer AL, Pearson SA, Dobbins TA, Er CC, Ward RL, Vajdic CM 2015, ‘Patterns of care and survival after a cancer of unknown primary (CUP) diagnosis: a population-based nested cohort study in Australian Government Department of Veterans’ Affairs clients.’ Cancer Epidemiol 39(4): 578–584.
Silver JK, Baima J 2013, ‘Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes.’ Am J Phys Med Rehabil 92(8): 715–727.
Sjoquist K, Zalcberg J 2013, ‘Clinical trials – advancing cancer care.’ Cancer Forum 37(1), viewed October 2012, <www.cancerforum.org. au/Issues/2013/March/Forum/ Clinical_trials. htm>.
Smith A, Bellizzi K, Keegan T, Zebrack B, Chen V, Neale A, et al. 2013, ‘Health-related quality of life of adolescent and young adult patients with cancer in the United States: the Adolescent and Young Adult Health Outcomes and Patient Experience Study.’ J Clin Oncol 31(17): 2136– 2145.
Steer B, Marx G, Singhal N, McJannett M, Goldstein D, Prowse R 2009, ‘Cancer in older people: a tale of two disciplines.’ Intern Med J 39: 771–775.
Taillibert S, Laigle-Donadey F, Sanson M 2004, ‘Palliative care in patients with primary brain tumours’, Current Opinion in Oncology, vol. 16, pp. 587–592.
Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA 2010, ‘Early palliative care for patients with non-metastatic nonsmall cell lung cancer.’ New Engl J Med 363(8): 733–742.
Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, et al. 2017, ‘Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial.’ J Clin Oncol 35(8): 834–841.
Tey J, Soon YY, Koh WY, Leong CN, Choo BA, Ho F, et al. 2017, ‘Palliative radiotherapy for gastric cancer: a systematic review and meta- analysis.’ Oncotarget 8(15): 25797–25805.
Tothill RW, Li J, Mileshkin L, Doig K, Siganakis T, Cowin P, et al. 2013, ‘Massively-parallel sequencing assists the diagnosis and guided treatment of cancers of unknown primary.’ J Pathol 231(4): 413–423.
Urban D, Rao A, Bressel M, Lawrence YR, Mileshkin L 2013, ‘Cancer of unknown primary: a population-based analysis of temporal change and socioeconomic disparities.’ Br J Cancer 109(5): 1318–1324.
Varghese AM, Arora A, Capanu M, Camacho N, Won HH, Zehir A, et al. 2017, ‘Clinical and molecular characterization of patients with cancers of unknown primary in the modern era.’ Ann Oncol 28(12): 3015–3021.
Vajdic CM, Goldstein D 2015, ‘Cancer of unknown primary site.’ Aust Fam Physician 44(9): 640–643.
Wagland R, Bracher M, Drosdowsky A, Richardson A, Symons J, Mileshkin L, et al. 2017, ‘Differences in experiences of care between patients diagnosed with metastatic cancer of known and unknown primaries: mixed-method findings from the 2013 cancer patient experience survey in England.’ BMJ Open 7(9): e017881.
Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighly N, Oza A, et al. 2014, ‘Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial.’ Lancet 383(9930): 1721–1730.







