Blood Cancer – Acute myeloid leukaemia
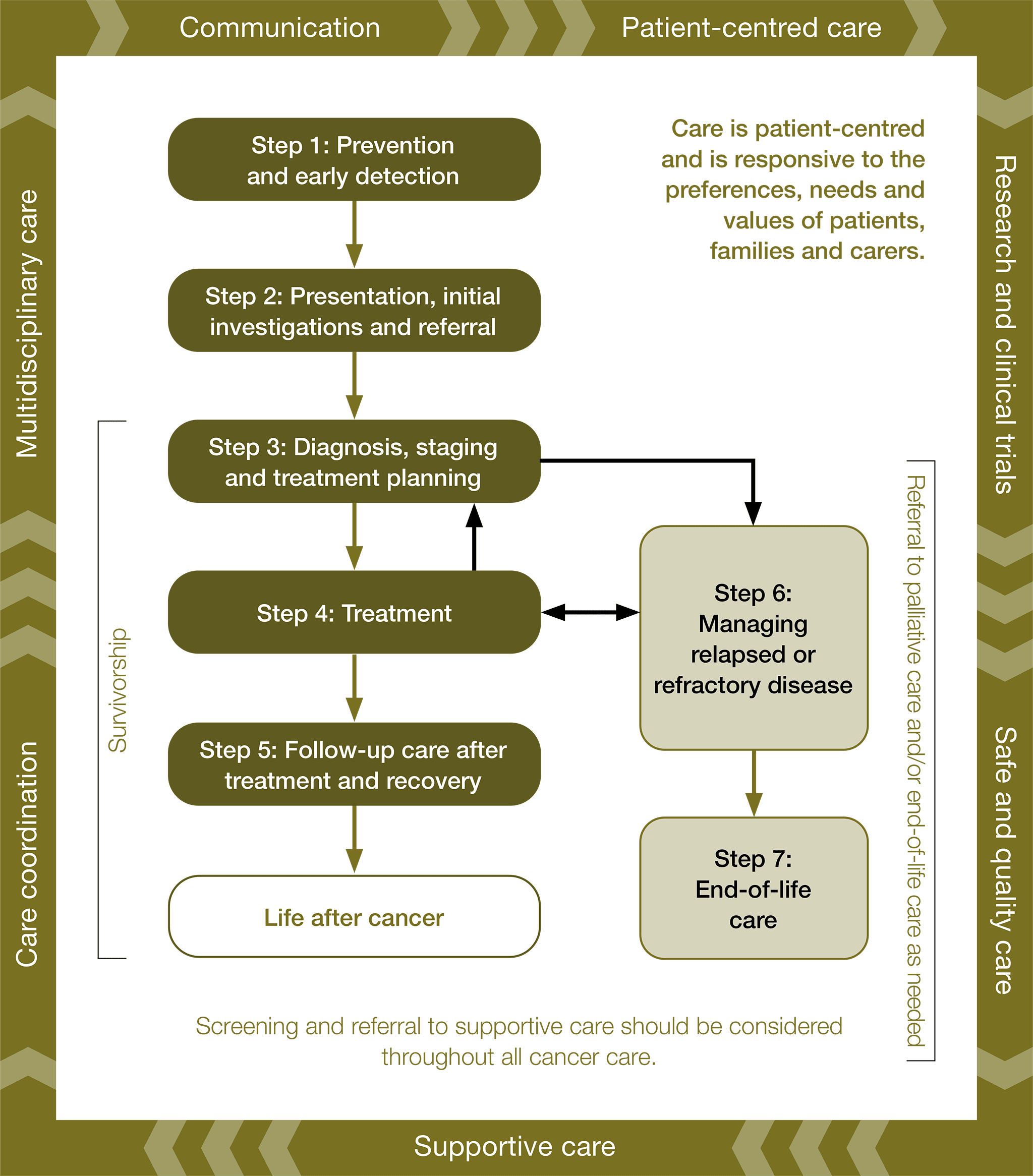
Optimal care pathways map seven key steps in cancer care. Each of these steps outlines nationally agreed best practice for the best level of care. While the seven steps appear in a linear model, in practice, patient care does not always occur in this way but depends on the particular situation (e.g. the type of cancer, when and how the cancer is diagnosed, prognosis, management, the patient’s decisions and their physiological response to treatment).
The principles underpinning optimal care pathways always put patients at the centre of care throughout their experience and prompt the healthcare system to deliver coordinated care.
The optimal care pathways do not constitute medical advice or replace clinical judgement, and they refer to clinical guidelines and other resources where appropriate.
Evidence-based guidelines, where they exist, should inform timeframes. Treatment teams need to recognise that shorter timeframes for appropriate consultations and treatment can promote a better experience for patients. Three steps in the pathway specify timeframes for care. They are designed to help patients understand the timeframes in which they can expect to be assessed and treated, and to help health services plan care delivery in accordance with expert-informed time parameters to meet the expectation of patients. These timeframes are based on expert advice from the Acute Myeloid Leukaemia Working Group.
Timeframes for care
|
Step in pathway |
Care point |
Timeframe |
|
Presentation, initial investigations and referral |
Signs and symptoms |
Presenting symptoms should be promptly and clinically triaged with a health professional |
|
Initial investigations initiated by GP |
The GP should begin investigations immediately if AML is suspected Laboratory results should be actively followed up and progressed on the same day |
|
|
Referral for emergency assessment/ Initial referral |
Patients with sepsis, bleeding or severe symptoms should be regarded as a medical emergency and be referred immediately to an appropriate emergency facility without necessarily waiting for results of laboratory tests (same day) Patients with a laboratory diagnosis of possible AML should be referred for an urgent assessment by a haematologist at an appropriate facility within 24 hours (unless advised otherwise by a haematologist) |
|
|
Diagnosis, staging and treatment planning |
Diagnosis and staging |
Morphological assessment to identify APL should be conducted immediately and the result conveyed to the treating physician as soon as possible For all patients with AML, other results necessary for immediate management decisions should be available within 72 hours of the patient presenting |
|
Multidisciplinary meeting and treatment planning |
Immediate treatment is often required before a full MDM ratifies the management plan details. Multidisciplinary input is likely to be required at several points after the first treatment begins |
|
|
Treatment |
Systemic therapy |
Induction therapy should start promptly once a diagnosis is made and a treatment plan for intensive chemotherapy is confirmed Consolidation therapy should start within 6 weeks of induction chemotherapy beginning |
|
Allogenic stem cell transplant |
Donor searches should begin for all anticipated allogeneic stem cell transplant (allo-SCT) canditates in first remission (CR1) patients as soon as their risk status is known |
|
|
Radiation therapy |
Radiation therapy may be used for symptom control in palliation and occasionally for treatment |
Seven steps of the optimal care pathway
Step 1: Prevention and early detection
Step 2: Presentation, initial investigations and referral
Step 3: Diagnosis, staging and treatment planning
Step 4: Treatment
Step 5: Care after initial treatment and recovery
Step 6: Managing relapsed or refractory disease
Step 7: End-of-life care
This pathway covers acute myeloid leukaemia (AML) in adults, including acute promyelocytic leukaemia (APL). AML is the most common form of acute leukaemia in adults (NCCN 2019). The yearly incidence rate of AML in Australian adults is 3.9 cases per 100,000, with a five-year survival rate of 28 per cent (AIHW 2019).
This step outlines recommendations for the prevention and early detection of AML. Except in uncommon specific circumstances (see section 1.2), the principal recommendations for most people are the same as for cancer in general.
Evidence shows that not smoking, avoiding or limiting alcohol intake, eating a healthy diet, maintaining a healthy body weight, being physically active, being sun smart and avoiding exposure to oncoviruses or carcinogens may help reduce cancer risk (Cancer Council Australia 2018).
The causes of AML are not fully understood, and there is currently no clear prevention strategy.
Most people have no identifiable risk factors. It is possible for AML to run in families but is uncommon. However, in a small proportion of patients, risk factors can be identified. Currently known risk factors include:
- advanced age
- prior chemotherapy, radiation therapy or high-dose radiation exposure
- a known previous haematological disorder with a risk of leukaemic transformation, such as myelodysplastic syndromes, myeloproliferative diseases or congenital neutropenic syndrome
- known predisposing genetic disorders such as Down syndrome, Trisomy 8, Bloom syndrome, Ataxia-telangiectasia, Diamond-Blackfan anaemia, Shwachman-Diamond syndromes, Li-Fraumeni syndrome, neurofibromatosis type 1, severe congenital neutropenia or Fanconi anaemia
- obesity
- tobacco smoking
- having a first-degree relative with AML
- environmental exposure of industrial chemicals such as benzene (ACS 2018).
In patients with pre-existing pre-leukaemic disorders (e.g. myelodysplasia, other myeloid neoplasms) and pre-disposing genetic disorders, routine care of these should include full blood counts and bone marrow biopsies at appropriate clinical intervals. This enables early detection in many circumstances. The frequency of blood tests and any bone marrow biopsies should be determined by standard frequency appropriate to the pre-existing or pre-disposing condition. For some conditions, such as myelodysplasia, validated risk assessment tools are available to guide practice in this regard.
There are no screening programs for AML.
This step outlines the process for the general practitioner to initiate the right investigations and refer to the appropriate specialist in a timely manner. The types of investigations the general practitioner undertakes will depend on many factors, including access to diagnostic tests, the availability of medical specialists and patient preferences.
Symptoms at presentation are usually non-specific. The following symptoms should be investigated:
- fatigue, pallor or other symptoms of anaemia
- symptoms of serious infection, such as tachycardia, high fevers, rigors
- unresolving or unusual infection or fever
- abnormal bleeding or bruising
- sore gums or mouth ulcers
- unexplained bone pain
- unintentional weight loss
- unexplained fevers.
The following signs and symptoms require consultation as a medical emergency:
- sepsis
- symptomatic anaemia
- severe thrombocytopenia < 20 × 109/L
- major laboratory abnormalities
- very high white cell count (> 50 × 109/L) or signs of hyperviscosity, such as visual disturbance, confusion, severe headache or breathlessness
- spontaneous/uncontrolled bleeding
- coagulopathy.
People with AML may only have mild symptoms. It is not uncommon that a patient with few or no symptoms is diagnosed unexpectedly on a blood test conducted in primary care.
The presence of multiple signs and symptoms listed above is highly suggestive of AML, particularly in people with a history of an underlying pre-disposing haematological condition.
Presenting symptoms should be promptly and clinically triaged with a health professional.
If a serious blood disorder is suspected, a focused medical history and thorough clinical assessment should be undertaken.
Full blood count and film should be performed immediately.
If the patient is clinically unwell (presents with symptomatic anaemia, spontaneous bleeding, sepsis and has symptoms of hyperviscosity), immediate referral to an emergency facility is recommended without waiting for blood results.
Pathology laboratories should directly contact the referring doctor if leukaemia is suspected (e.g. unexplained pancytopenia or blasts detected in the blood). Results should be actively followed up by the general practitioner and acted upon on the same day. Morphologic evidence of APL, disseminated intravascular coagulation, severe thrombocytopenia and any organ dysfunction (renal/liver failure) should be considered a medical emergency.
Patients with a laboratory diagnosis of possible AML should be referred for immediate assessment by a haematologist at an appropriate facility.
The general practitioner should begin investigations immediately if AML is suspected.
Laboratory results should be actively followed up and progressed on the same day. It is the responsibility of both the referring doctor and pathology laboratory to identify the possibility of a diagnosis of AML and take appropriate action.
If the general practitioner confirms, or suspects a diagnosis of AML but cannot confirm it, they must refer the patient to see a specialist (haematologist) to make the diagnosis.
Haematologists must expedite assessments for referred patients. Healthcare providers should facilitate patients’ rapid access to acute leukaemia treatment services. All patients with suspected AML should be evaluated and cared for by a multidisciplinary team with experience in managing AML. Readily accessible contact referral details for leukaemia treatment centres should be available.
Patients should be enabled to make informed decisions about their choice of specialist and health service. General practitioners should make referrals in consultation with the patient after considering the clinical care needed, cost implications (see referral options and informed financial consent), waiting periods, location and facilities, including discussing the patient’s preference for health care through the public or the private system.
Referral for suspected or diagnosed AML should include the following essential information to accurately triage and categorise the level of clinical urgency:
- important psychosocial history and relevant medical history
- family history, current symptoms, medications and allergies
- results of current clinical investigations (imaging and pathology reports)
- results of all prior relevant investigations
- notification if an interpreter service is required.
Many services will reject incomplete referrals, so it is important that referrals comply with all relevant health service criteria.
If access is via online referral, a lack of a hard copy should not delay referral.
The specialist should provide timely communication to the general practitioner about the consultation and should notify the general practitioner if the patient does not attend appointments.
Aboriginal and Torres Strait Islander patients will need a culturally appropriate referral. To view the optimal care pathway for Aboriginal and Torres Strait Islander people and the corresponding quick reference guide, visit the Cancer Australia website. Download the consumer resources – Checking for cancer and Cancer from the Cancer Australia website.
- Patients with sepsis, bleeding or severe symptoms should be regarded as a medical emergency and be referred immediately to an appropriate emergency facility without necessarily waiting for results of laboratory tests (same day). All emergency facilities should have existing arrangements to receive urgent haematological advice.
- Patients with suspected AML who present to an emergency department should be triaged as a medical emergency initially and discussed immediately with a clinical haematology service and/or transferred immediately to a specialist centre. This particularly applies to patients with suspected APL.
- Patients with a laboratory diagnosis of possible AML should be referred for an urgent assessment by a haematologist at an appropriate facility within 24 hours. A deferred assessment should only be done after a discussion between the referring doctor and the responsible haematologist.
The patient’s general practitioner should consider an individualised supportive care assessment where appropriate to identify the needs of an individual, their carer and family. Refer to appropriate support services as required. See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific needs may arise for patients at this time:
- assistance for dealing with the emotional distress and/or anger of dealing with a potential cancer diagnosis, anxiety/depression, interpersonal problems and adjustment difficulties
- weight loss, which may require a nutritional assessment.
For more information refer to the National Institute for Health and Care Excellence 2015 guidelines, Suspected cancer: recognition and referral.
For additional information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
The general practitioner is responsible for:
- providing patients with information that clearly describes to whom they are being referred, the reason for referral and the expected timeframes for appointments
- requesting that patients notify them if the specialist has not been in contact within the expected timeframe
- considering referral options for patients living rurally or remotely
supporting the patient while waiting for the specialist appointment (Cancer Council nurses are available to act as a point of information and reassurance during the anxious period of awaiting further diagnostic information; patients can contact 13 11 20 nationally to speak to a cancer nurse).
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
Step 3 outlines the process for confirming the diagnosis and stage of cancer and for planning subsequent treatment. The guiding principle is that interaction between appropriate multidisciplinary team members should determine the treatment plan.
The treatment team, after taking a thorough medical history and making a thorough medical examination of the patient, should undertake the following investigations under the guidance of a specialist.
Diagnostic evaluation is required for two complementary purposes – first, to establish a precise diagnosis according to the most recent classification system, and second, to assess the presence and management of comorbidities and the patient’s fitness because these affect both response to treatment and toxicity from treatment.
To achieve this, the treatment team should:
- perform a thorough physical examination, including assessing for the presence of extramedullary disease (e.g. leukaemia cutis, gum infiltration and/or central nervous system symptoms)
- undertake the following investigations under the guidance of a specialist:
- peripheral blood tests
- bone marrow aspirate (BMA)
- trephine biopsy +/- lumbar puncture, imaging or tissue biopsy when extramedullary disease is suspected.
Note: Details of specific tests appear below in section 3.2.
Pathology specimens should be collected and reviewed by a pathologist with expertise in diagnosing AML, before a treatment plan is instituted.
Where safe and timely to do so, it is preferable that the diagnostic blood tests and bone marrow biopsy are performed at the specialist treatment centre. This will facilitate review of blood and bone marrow results by the specialist management team and ensure all necessary tests are conducted.
Morphological assessment to identify APL should be conducted immediately and the result conveyed to the treating physician as soon as possible.
For all patients with AML, other results necessary for immediate management decisions should be available within 72 hours of the patient presenting.
Specialised testing is performed to:
- ensure accurate diagnoses
- accurately sub-classify AML
- inform prognosis
- inform treatment decisions that are evidence-based.
Classification is the principle process by which key information is collated to inform prognosis and management of patients with AML.
Classification and risk stratification for AML involves these tests:
- morphological assessment
- cytogenetics
- flow cytometry
- molecular pathology (genetic testing).
AML is classified according to the World Health Organization’s classification of AML tumours (Arber et al. 2016). The European LeukemiaNet stratification system classifies patients as having favourable, intermediate and adverse risk based on karyotype, selected molecular abnormalities (currently FLT3, NPM1, TP53, ASXL1, RUNX1 and CEBPA mutation status) (Döhner et al. 2017).
The other most important prognostic features are age at diagnosis, performance status, presence of extramedullary disease, hyperleukocytosis, therapy-related AML (previous exposure to cytotoxics), presence of an antecedent bone marrow failure syndrome and response to induction chemotherapy.
Newer molecular markers with prognostic and therapeutic relevance in AML are likely to become clinically routine in the near future (Grimwade et al. 2016).
Most genetic abnormalities in AML only occur in abnormal blood cells and are not related to genetic abnormalities that affect the whole body and/or are inherited. However, heritable genetic abnormalities may be identifiable in a very small number of patients. AML with a genetic predisposition is an entity in the World Health Organization classification, and most diagnostic centres have access to identification of heritable genetic abnormalities related to leukaemia. This becomes significantly relevant if a family member is being considered as a stem cell donor.
It is important to evaluate and document relevant organ functions (e.g. respiratory, cardiac, hepatic, renal) and physiological robustness using validated assessment tools for all patients, especially older patients (Sorror et al. 2017).
Careful clinical and haematological assessment is required to identify patients in whom the start of chemotherapy could or should be delayed. The presence of an active infection at diagnosis is important to identify.
In addition to a clinical examination, the following investigations/procedures are recommended:
- coagulation status to detect leukemia-related coagulopathy (Döhner et al. 2017)
- MRI brain +/– lumbar puncture if central nervous system involvement is suspected
- CT/PET scan to help assess for extramedullary disease where this is clinically suspected
- cardiac investigation including an ECHO or a gated heart pool scan in patients being considered for induction therapy
- human leukocyte antigen (HLA) typing and HLA antibody screening at diagnosis in patients being considered for induction therapy.
Each unit should have a policy about if, and when, HLA typing of available first- and second-degree family members should occur. This policy should be agreed with the allo-SCT unit to which referrals are usually directed.
In patients with adverse or intermediate risk disease, early allo-SCT should be considered (Döhner et al. 2010) and, therefore, a donor search should be carried out as early as possible in accordance with agreed policies of the allo-SCT unit to which referrals are usually directed.
Patient performance status is a central factor in cancer care and should be clearly documented in the patient’s medical record.
Performance status should be measured and recorded using an established scale such as the Karnofsky scale or the Eastern Cooperative Oncology Group (ECOG) scale.
Because of the urgency and complexity of treatment, every clinical haematology unit should have predefined, peer-reviewed treatment models of care that have been endorsed by the multidisciplinary team. Assessment of the premorbid state is an essential component of the treatment planning process.
Prevention and management of infections in AML include the following:
- All patients should undergo screening for infections at high risk of reactivation or transmission before beginning treatment.
- Some infections are determined by epidemiological risk of exposure and history of recent travel and/or extended habitation in high-risk countries.
- Minimum requirements would include cytomegalovirus, hepatitis B, hepatitis C and HIV screening. Other tests such as for tuberculosis, strongyloides serology, and screening for multidrug-resistant pathogens as per institutional policy, should be considered.
- Patients with antibiotic allergy labels should have suspected allergies reassessed where possible.
- All institutions should have empiric sepsis guidelines/pathways that include appropriate recommendations for the initial management of neutropenic fever. Specialists in infectious diseases may be required for advice about duration and appropriate antibiotics based on pathogens isolated and patient factors (allergy, renal impairment).
- Vaccination status should be assessed for all patients. Vaccination with influenza and Streptococcus pneumoniae can recommence after three months if in complete remission.
- Prophylaxis guidelines for fungal and viral infections should accord with published national guidelines.
A number of factors should be considered at this stage:
- the patient’s overall condition, life expectancy, personal preferences and decision- making capacity
- discussing the multidisciplinary team approach to care with the patient
- appropriate and timely referral to an MDM
- pregnancy and fertility
- support with travel and accommodation
- teleconferencing or videoconferencing as required.
Induction treatment is often required before a full MDM ratifies details of the ongoing management plan (which should include full details of the response assessment). Most patients will receive their initial treatment as inpatients, allowing their initial multidisciplinary treatment planning to be established on the ward. For patients undergoing induction chemotherapy, presentation to, and consideration within, an MDM is most important once the outcome of the induction therapy is known. At this point, a review of the patient is required to inform further management and supportive care needs.
For patients not eligible for induction chemotherapy, or where uncertainty of the approach exists, a review at an MDM should occur as soon as practicable (before definitive treatment), to establish the recommended treatment plan and all aspects of supportive care, including early preparation for the post-treatment phase.
The level of discussion may vary, depending on the patient’s clinical and supportive care factors. Some patients with non-complex cancers may not be discussed by a multidisciplinary team; instead the team may have treatment plan protocols that will be applied if the patient’s case (cancer) meets the criteria. If patients are not discussed at an MDM, they should at least be named on the agenda for noting. The proposed treatment must be recorded in the patient’s medical record and should be recorded in an MDM database where one exists.
Teams may agree on standard treatment protocols for non-complex care, facilitating patient review (by exception) and associated data capture.
Results of all relevant tests and access to images should be available for the MDM. Information about the patient’s concerns, preferences and social and cultural circumstances should also be available.
The multidisciplinary team requires administrative support in developing the agenda for the meeting, for collating patient information and to ensure appropriate expertise around the table to create an effective treatment plan for the patient. The MDM has a chair and multiple lead clinicians. Each patient case will be presented by a lead clinician (usually someone who has seen the patient before the MDM). In public hospital settings, the registrar or clinical fellow may take this role. A member of the team records the outcomes of the discussion and treatment plan in the patient history and ensures these details are communicated to the patient’s general practitioner. The team should consider the patient’s values, beliefs and cultural needs as appropriate to ensure the treatment plan is in line with these. There may be early consideration of post-treatment pathways at this point – for example, shared follow-up care.
The multidisciplinary team should be composed of the core disciplines that are integral to providing good care. Team membership should reflect both clinical and supportive care aspects of care. Pathology expertise is essential.
See ‘About this OCP’ for a list of team members who may be included in the multidisciplinary team for AML.
Core members of the multidisciplinary team are expected to attend most MDMs either in person or remotely via virtual mechanisms. Additional expertise or specialist services may be required for some patients. An Aboriginal and Torres Strait Islander cultural expert should be considered for all patients who identify as Aboriginal or Torres Strait Islander.
The general practitioner who made the referral is responsible for the patient until care is passed to another practitioner who is directly involved in planning the patient’s care.
The general practitioner may play a number of roles in all stages of the cancer pathway including diagnosis, referral, treatment, shared follow-up care, post-treatment surveillance, coordination and continuity of care, as well as managing existing health issues and providing information and support to the patient, their family and carer.
A nominated contact person from the multidisciplinary team may be assigned responsibility for coordinating care in this phase. Care coordinators are responsible for ensuring there is continuity throughout the care process and coordination of all necessary care for a particular phase (COSA 2015). The care coordinator may change over the course of the pathway.
The lead clinician is responsible for overseeing the activity of the team and for implementing treatment within the multidisciplinary setting.
Participation in clinical trials, registries and tissue banking, where available, is considered a standard of care for patients with AML. Cross-referral between clinical trials centres should be encouraged to facilitate participation.
For more information visit:
Cancer prehabilitation uses a multidisciplinary approach combining exercise, nutrition and psychological strategies to prepare patients for the challenges of cancer treatment such as surgery, systemic therapy and radiation therapy. Team members may include anaesthetists, oncologists, surgeons, haematologists, clinical psychologists, exercise physiologists, physiotherapists and dietitians, among others.
Patient performance status is a central factor in cancer care and should be frequently assessed. All patients should be screened for malnutrition using a validated tool, such as the Malnutrition Screening Tool (MST). The lead clinician may refer obese or malnourished patients to a dietitian preoperatively or before other treatments begin.
Patients who currently smoke should be encouraged to stop smoking before receiving treatment. This should include an offer of referral to Quitline in addition to smoking cessation pharmacotherapy if clinically appropriate.
Evidence indicates that patients who respond well to prehabilitation may have fewer complications after treatment. For example, those who were exercising before diagnosis and patients who use prehabilitation before starting treatment may improve their physical or psychological outcomes, or both, and this helps patients to function at a higher level throughout their cancer treatment (Cormie et al. 2017; Silver 2015).
For patients with AML, the multidisciplinary team should consider these specific prehabilitation assessments and interventions for treatment-related complications or major side effects:
- conducting a physical and psychological assessment to establish a baseline function level
- identifying impairments and providing targeted interventions to improve the patient’s function level (Silver & Baima 2013)
- reviewing the patient’s medication to ensure optimisation and to improve adherence to medicine used for comorbid conditions.
Following completion of primary cancer treatment, rehabilitation programs have considerable potential to enhance physical function.
Cancer and cancer treatment may cause fertility problems. This will depend on the age of the patient, the type of cancer and the treatment received. Infertility can range from difficulty having a child to the inability to have a child. Infertility after treatment may be temporary, lasting months to years, or permanent (AYA Cancer Fertility Preservation Guidance Working Group 2014).
Patients need to be advised about and potentially referred for discussion about fertility preservation before starting treatment and need advice about contraception before, during and after treatment. Patients and their family should be aware of the ongoing costs involved in optimising fertility. Fertility management may apply in both men and women. Fertility preservation options are different for men and women. Fertility preservation procedures may not always be feasible in patients with newly diagnosed AML, which can require immediate chemotherapy. The need for ongoing contraception applies to both men and women.
The potential for impaired fertility should be discussed and reinforced at different time points as appropriate throughout the diagnosis, treatment, surveillance and survivorship phases of care. These ongoing discussions will enable the patient and, if applicable, the family to make informed decisions. All discussions should be documented in the patient’s medical record.
See the Cancer Council website for more information.
Rehabilitation may be required at any point of the care pathway. If it is required before treatment, it is referred to as prehabilitation (see section 3.6.1).
All members of the multidisciplinary team have an important role in promoting rehabilitation. Team members may include occupational therapists, speech pathologists, dietitians, social workers, psychologists, physiotherapists, exercise physiologists and rehabilitation specialists.
To maximise the safety and therapeutic effect of exercise for people with cancer, all team members should recommend that people with cancer work towards achieving, and then maintaining, recommended levels of exercise and physical activity as per relevant guidelines. Exercise should be prescribed and delivered under the direction of an accredited exercise physiologist or physiotherapist with experience in cancer care (Vardy et al. 2019). The focus of intervention from these health professionals is tailoring evidence-based exercise recommendations to the individual patient’s needs and abilities, with a focus on the patient transitioning to ongoing self-managed exercise.
Other issues that may need to be dealt with include managing cancer-related fatigue, improving physical endurance, achieving independence in daily tasks, optimising nutritional intake, returning to work and ongoing adjustment to cancer and its sequels. Referrals to dietitians, psychosocial support, return-to-work programs and community support organisations can help in managing these issues.
The lead or nominated clinician should take responsibility for these tasks:
- discussing treatment options with patients and carers, including the treatment intent and expected outcomes, and providing a written version of the plan and any referrals
- providing patients and carers with information about the possible side effects of treatment, managing symptoms between active treatments, how to access care, self-management strategies and emergency contacts
- encouraging patients to use question prompt lists and audio recordings, and to have a support person present to aid informed decision making
- initiating a discussion about advance care planning and involving carers or family if the patient wishes.
See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific challenges and needs may arise for patients at this time:
- assistance for dealing with psychological and emotional distress while adjusting to the diagnosis; treatment phobias; existential concerns; stress; difficulties making treatment decisions; anxiety or depression or both; psychosexual issues such as potential loss of fertility and premature menopause; history of sexual abuse; and interpersonal problems
- management of physical symptoms such as pain and fatigue (Australian Adult Cancer Pain Management Guideline Working Party 2019)
- malnutrition or undernutrition, identified using a validated nutrition screening tool such as the MST (note that many patients with a high BMI [obese patients] may also be malnourished [WHO 2018])
- patients with a high or very high body mass index should have chemotherapy dosed as per agreed upon guidelines (Griggs et al. 2012) – specialty pharmacy advice may also be required concerning dosing of other pharmaceuticals in this patient group
- access to peer support
- support for families or carers who are distressed with the patient’s cancer diagnosis
- support for families/relatives who may be distressed after learning of a genetically linked cancer diagnosis
- specific spiritual needs that may benefit from the involvement of pastoral/spiritual care.
Additionally, palliative care may be required at this stage.
For more information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
In discussion with the patient, the lead clinician should undertake the following:
- establish if the patient has a regular or preferred general practitioner and if the patient does not have one, then encourage them to find one
- provide written information appropriate to the health literacy of the patient about the diagnosis and treatment to the patient and carer and refer the patient to the Guide to best cancer care (consumer optimal care pathway) for AML, as well as to relevant websites and support groups as appropriate
- offer all patients of childbearing years undergoing allo-SCT the opportunity of preserving their fertility prior to treatment (referral to fertility counselling may be appropriate as discussed in section 3.6.2)
- provide a treatment care plan including contact details for the treating team and information on when to call the hospital
- discuss a timeframe for diagnosis and treatment with the patient and carer
- discuss the benefits of multidisciplinary care and gain the patient’s consent before presenting their case at an MDM
- provide brief advice and refer to Quitline (13 7848) for behavioural intervention if the patient currently smokes (or has recently quit), and prescribe smoking cessation pharmacotherapy, if clinically appropriate
- recommend an ‘integrated approach’ throughout treatment regarding nutrition, exercise and minimal or no alcohol consumption among other considerations
- communicate the benefits of continued engagement with primary care during treatment for managing comorbid disease, health promotion, care coordination and holistic care
- where appropriate, review fertility needs with the patient and refer for specialist fertility management (including fertility preservation, contraception, management during pregnancy and of future pregnancies)
- be open to and encourage discussion about the diagnosis, prognosis (if the patient wishes to know) and survivorship and palliative care while clarifying the patient’s preferences and needs, personal and cultural beliefs and expectations, and their ability to comprehend the communication
- encourage the patient to participate in advance care planning including considering appointing one or more substitute decision-makers and completing an advance care directive to clearly document their treatment preferences. Each state and territory has different terminology and legislation surrounding advance care directives and substitute decision-makers.
The lead clinician has these communication responsibilities:
- involving the general practitioner from the point of diagnosis
- ensuring regular and timely communication with the general practitioner about the diagnosis, treatment plan and recommendations from MDMs and inviting them to participate in MDMs (consider using virtual mechanisms)
- supporting the role of general practice both during and after treatment
- discussing shared or team care arrangements with general practitioners or regional cancer specialists, or both, together with the patient.
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
Step 4 describes the optimal treatments for AML, the training and experience required of the treating clinicians and the health service characteristics required for optimal cancer care.
All health services must have clinical governance systems that meet the following integral requirements:
- identifying safety and quality measures
- monitoring and reporting on performance and outcomes
- identifying areas for improvement in safety and quality (ACSQHC 2020).
The resources below serve as guidelines. Treatment plans for patients should be individualised and discussed in MDMs. The choice of treatments depends on the registration and reimbursement status of the drugs and the availability of clinical trials.
- National Comprehensive Cancer Network, 2019, Acute myeloid leukemia, Version 3.2019
- National Cancer Institute 2020, Adult acute myeloid leukemia treatment (PDQ) – health professional version
- European Society for Medical Oncology 2020, Clinical practice guidelines – myeloid leukaemia in adult patients
- European LeukemiaNet, ‘Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel’
The intent of treatment can be defined as one of the following:
- curative
- anti-leukaemia therapy to improve longevity and quality of life without expectation of cure
- symptom palliation including active supportive care.
The treatment intent should be documented in the patient’s medical record. Achieving a complete remission is the first goal for patients receiving either curative intent therapy or treatment designed to improve longevity and quality of life without expectation of cure.
The potential benefits need to be balanced against the morbidity and risks of treatment.
The lead clinician should discuss the advantages and disadvantages of each treatment and associated potential side effects with the patient and their carer or family before treatment consent is obtained and begins so the patient can make an informed decision. Supportive care services should also be considered during this decision-making process. Patients should be asked about their use of (current or intended) complementary therapies (see Appendix D).
Timeframes for starting treatment should be informed by evidence-based guidelines where they exist. The treatment team should recognise that shorter timeframes for appropriate consultations and treatment can promote a better experience for patients.
Initiate advance care planning discussions with patients before treatment begins (this could include appointing a substitute decision-maker and completing an advance care directive). Formally involving a palliative care team/service may benefit any patient, so it is important to know and respect each person’s preference (AHMAC 2011).
Patients fit for intensive chemotherapy
Systemic chemotherapy for AML is a key component of treatment and is divided into two phases: induction therapy to achieve complete remission; and consolidation therapy once a remission has been achieved to maintain ongoing remission or as a bridge to curative treatment – that is, an allogeneic bone marrow transplant (also known as stem cell transplant).
Induction chemotherapy should ideally only be started when all diagnostic criteria have been satisfied (Döhner et al. 2017). In patients with suspected APL and hyperleukocytosis, the risk of severe complications is high and chemotherapy poses the risk of worsening disseminated intravascular coagulation. Leukopheresis is contraindicated in this scenario. Starting differentiation therapy immediately needs to be considered in consultation with an expert in this area. Treatment with ATRA is frequently initiated on the suspicion of APL. In these circumstances, emergency therapy may be required before completing diagnostic sampling.
All patients undergoing intensive chemotherapy will need a central intravenous line inserted (with platelet transfusion and correction of coagulopathy if necessary). Such devices should only be inserted by proceduralists experienced in such procedures.
After recovering from induction therapy, it is important to assess the response to initial treatment, including complications (e.g. the severity of side effects and sepsis), in order to plan future therapy. Patients who fail to achieve remission have a poor prognosis (McMahon & Perl 2019), while the outcome for patients in remission depends on subsequent therapy.
Once patients are in remission, consolidation therapy is always indicated when cure is the intention (Döhner et al. 2010). Following induction therapy, additional treatment should be given because the median disease-free survival for patients who receive no additional therapy is only four to eight months (Cassileth et al. 1998). The aim of consolidation therapy is to prevent relapse with maximal efficiency and minimal toxicity. Current approaches to induction and consolidation therapy include short-term, relatively intensive chemotherapy, or high-dose chemotherapy (summarised in Döhner et al. 2015). There is no consensus on a single ‘best’ post-remission treatment schedule, nor the optimal number of cycles of consolidation chemotherapy.
Consequently, consolidation therapy for AML patients who have achieved complete remission is determined after considering a combination of the following factors:
- the patient’s age and fitness
- prognosis
- tolerance of prior therapy
- minimal (also called measurable) residual disease (MRD) status in selected AML subtypes
- whether the patient is a candidate for an allogeneic stem cell transplant.
Patients not fit for intensive chemotherapy
New therapies are starting to emerge that may offer meaningful clinical activity in patients considered unfit for intensive chemotherapy. This may not necessarily be based on age. Referral to a clinical trial should be a priority. Available current treatment options include low-dose chemotherapy or hypomethylating agents for older patients (> 75 years) or for patients with significant comorbidities. Alternatively, palliative/supportive care therapy to control symptoms will be appropriate for some people. Emerging therapies, including those not currently approved by the Therapeutic Goods Administration (TGA) or reimbursed in Australia, are outlined in the National Comprehensive Cancer Network’s clinical guidelines (NCCN 2019).
Timeframe for starting treatment
- Induction therapy should start promptly once a diagnosis is made and a treatment plan for intensive chemotherapy is confirmed.
- Consolidation therapy should start within six weeks of induction chemotherapy beginning.
Allogeneic stem cell transplant
Potential candidates for allo-SCT (scheduled for the consolidation phase) should be identified at diagnosis. These considerations can change based on the patient’s response to initial treatment, overall tolerance and complications of subsequent treatment. A formal recommendation to proceed to allo-SCT should only occur after discussion at a focused bone marrow transplant MDM. Factors that need consideration include disease prognosis (incorporating response to treatment), comorbidities and functional status, and availability of suitable donor(s). Part of this assessment should include a formal haematopoietic cell transplantation (HCT)-comorbidity assessment.
Allo-SCT should be considered for:
- all younger patients depending on prognostic factors and patient preferences
- patients with non-favourable AML in first remission who have an acceptable allogeneic donor(s), noting that only a proportion of patients will benefit
- some patients whose disease fails to go into remission with intensive chemotherapy
- patients with rising MRD
- selected patients beyond CR1.
For patients with good-prognosis AML in their first complete remission (APL, core-binding factor AML, CEBPA with double mutation and NPM1 mutation in the absence of FLT3-ITD), the risks of allo-SCT exceed the benefits and a survival advantage has not been proven, especially if patients have low or absent levels of MRD after achieving remission.
Autografting may be appropriate for patients with relapsed acute promyelocytic leukemia in second molecular remission (Ganzel et al. 2016; Holter Chakrabarty et al. 2014; Lengfelder et al. 2015). Autograft may be considered in select favourable and intermediate-risk AML patients in stringent first remission in the absence of a suitable allogeneic donor (Gorin et al. 2008; Venditti et al. 2019). The role of autografting in managing other forms of AML is contentious. Autografting in these circumstances should be carried out in a clinical trial.
Timeframes for starting treatment
- Donor searches should begin for all anticipated allo-SCT candidates in first remission (CR1) patients as soon as the patient’s risk status is known.
- Individual treating units should ensure referral pathways for transplantation are established to minimise delays. Rapid-access pathways are required for patients for whom urgent transplantation may be appropriate.
Radiation therapy
Radiation therapy may be used for symptom control in palliation and occasionally to treat extramedullary disease.
Total body irradiation (TBI) may also be indicated as part of conditioning for allo-SCT and should only be given in centres with appropriately qualified and experienced staff and equipment.
Treatment of APL differs in several important aspects from therapy of all other AML types. The presentation of APL is a medical emergency because of the high risk of death as a result of the associated coagulopathy. Rapid initiation of APL-specific therapy is essential and, in some cases, may precede formal confirmation of the diagnosis. Treating units must have protocols for intensive supportive care including guidelines for blood product administration in managing coagulopathy.
Patients should undergo molecular monitoring after treatment to guide further therapy.
Resistance to therapy (refractory AML) is the major cause of treatment failure, rather than mortality due to infections and other treatment-related complications. Patients failing to respond to one or two cycles of induction treatment can be considered chemotherapy refractory and are at very high risk of ultimate treatment failure. In this circumstance other alternatives should be explored (non-chemotherapy options or clinical trials). While there are no standard salvage regimens for AML, intensive salvage chemotherapy can result in a second remission in approximately 55 per cent of patients aged 16–49, of which approximately two-thirds can then proceed to an allo-SCT (Döhner et al. 2017).
Patients offered an allo-SCT are carefully selected and must have an appropriately HLA-matched donor. It should be noted that patients with refractory disease who undergo an allo-SCT have limited chances of success and considerable morbidity from this procedure. For patients unsuited to this approach, palliative systemic treatment is often a reasonable option with limited toxic effects (Döhner et al. 2010).
Currently, midostaurin is the only Pharmaceutical Benefits Scheme (PBS)-listed targeted therapy approved to be used in combination with induction chemotherapy for newly diagnosed AML with a FLT3 internal tandem duplication (FLT3-ITD) or tyrosine kinase domain-activating mutation (FLT3-TKD) (DHS 2020; eviQ 2019b).
Enasidenib is a targeted oral therapy used as a single agent for treating refractory or relapsed IDH2-mutated AML. This drug has provisional TGA approval but is not PBS listed (February 2021). A phase 3 randomised trial of enasidenib or placebo in combination with induction chemotherapy for newly diagnosed IDH-mutated AML began in Australia in 2020. This trial also includes a randomisation of patients with IDH1-mutated AML to induction chemotherapy with or without the targeted IDH1 inhibitor ivosidenib.
Gilteritinib is a second-generation FLT3-inhibitor, approved by the TGA for patients with relapsed or refractory FLT3-mutant AML. This drug is not listed on the PBS (February 2021). It is administered as a single agent. A randomised trial of gilteritinib versus standard therapy (ADMIRAL) showed a survival benefit for patients receiving gilteritinib (Perl et al. 2019). A randomised trial of gilteritinib versus midostaurin in combination with induction chemotherapy in newly diagnosed FLT3-mutated AML began in Australia in 2020.
No immunotherapy drugs are TGA approved for treating AML.
A number of emerging therapies are being investigated for AML. Therapies that show promise for treating AML include novel targeted therapies, epigenetic therapies, immunotherapies and cell therapies (Davis et al 2018; DiNardo & Wei 2020; Wingelhofer & Somervaille 2019). These novel therapies are in various stages of clinical trial development and assessment. It is anticipated that some will become TGA-approved in the coming years.
The following training and experience is required of the appropriate specialist(s):
- Haematologists, radiation oncologists or medical oncologists (FRACP or equivalent) must have adequate training and experience with institutional credentialing and agreed scope of practice within this area (ACSQHC 2015).
- Nurses must have adequate training in systemic therapy administration, specialised nursing care for patients undergoing cancer treatments, including side effects and symptom management, and handling and disposal of cytotoxic waste.
- Interventional radiology and/or certified proceduralists must be competent in inserting central venous access devices.
- Systemic therapy should be prepared by a pharmacist with adequate training in systemic therapy medication, including dosing calculations according to protocols, formulations and/or preparation.
In a setting where no haematologist or medical oncologist is locally available (e.g. regional or remote areas), some components of less complex therapies may be delivered by a general practitioner or nurse with training and experience that enables credentialing and agreed scope of practice within this area. This should be in accordance with a detailed treatment plan or agreed protocol, and with communication as agreed with the medical oncologist or as clinically required.
Hospital or treatment unit characteristics for providing safe and quality care include:
- dedicated standard isolation rooms (single rooms with ensuite and clinical handwashing facilities)
- access to a cell separator for collecting peripheral blood progenitor cells
- HEPA-filtered environment/rooms in the inpatient setting
- immediate blood product support
- a clearly defined path to emergency care and advice after hours
- access to total parenteral nutrition
- access to a dental service familiar with mouth care issues experienced by haematology patients
- accessible emergency apheresis for managing hyperleukocytosis
- access to diagnostic pathology including basic haematology and biochemistry, and imaging
- rapid access to an interventional radiologist/proceduralist
- an infectious disease specialist
- cytotoxic drugs prepared in a pharmacy with appropriate facilities
- occupational health and safety guidelines regarding handling of cytotoxic drugs, including preparation, waste procedures and spill kits (eviQ 2019a)
- guidelines and protocols to deliver treatment safely (including dealing with extravasation of drugs)
- timely access to pathology
- coordination for combined therapy with radiation therapy, especially where facilities are not co-located.
Radiation oncology centre characteristics for providing safe and quality care include:
- linear accelerator (LINAC) capable of image-guided radiation therapy (IGRT)
- staff to be familiar with AML-specific radiation therapy techniques
- TBI-based preparative regimens only being delivered in centres with experience using TBI conditioning and autologous/allogeneic transplantation (minimum 10 procedures per year)
- dedicated CT planning
- access to MRI and PET imaging
- automatic record-verify of all radiation treatments delivered
- a treatment planning system
- trained medical physicists, radiation therapists and nurses with radiation therapy experience
- coordination for combined therapy with systemic therapy, especially where facilities are not co-located
- participation in Australian Clinical Dosimetry Service audits
- an incident management system linked with a quality management system.
Centres that do not have sufficient caseloads (for TBI and for overall management) should refer cases to a high-volume centre.
Early referral to palliative care can improve the quality of life for people with cancer and in some cases may be associated with survival benefits (Haines 2011; Temel at al. 2010; Zimmermann et al. 2014). This is particularly true for poor-prognosis AML to ensure optimal symptom control from treatment toxicity or progressive disease.
The lead clinician should ensure patients receive timely and appropriate referral to palliative care services. Referral should be based on need rather than prognosis. Emphasise the value of palliative care in improving symptom management and quality of life to patients and their carers.
The ‘Dying to Talk’ resource may help health professionals when initiating discussions with patients about future care needs (see ‘More information’). Ensure that carers and families receive information, support and guidance about their role in palliative care (Palliative Care Australia 2018).
Patients, with support from their family or carer and treating team, should be encouraged to consider appointing a substitute decision-maker and to complete an advance care directive.
Refer to step 6 for a more detailed description of managing patients with recurrent, residual or metastatic disease.
These online resources are useful:
The team should support the patient to participate in research or clinical trials where available and appropriate. Many emerging treatments are only available on clinical trials that may require referral to certain trial centres.
For more information visit the Cancer Australia website
See validated screening tools mentioned in Principle 4 ‘Supportive care’.
Therapy is often associated with a number of symptoms and physiological abnormalities. Patients are highly susceptible to infection from prolonged neutropenia and this can result in significant comorbidity and mortality. Patient education and strict adherence to universal neutropenic guidelines, access to infection control specialists, close monitoring of full blood count, and early intervention of neutropenic sepsis is essential. Patients need to be educated on the importance of personal hygiene and particularly dental care to minimise infection. Antimicrobial therapy including antiviral, antifungal and antibiotic therapy is often administered prophylactically to reduce risk of infections.
A number of specific challenges and needs may arise for patients at this time:
- assistance for dealing with emotional and psychological issues, including body image concerns, fatigue, quitting smoking, traumatic experiences, existential anxiety, treatment phobias, anxiety/depression, interpersonal problems and sexuality concerns
- potential isolation from normal support networks, particularly for rural patients who are staying away from home for treatment
- side effects resulting from high-dose therapy including alopecia, fatigue, cytopenias, mucositis (oral and bowel), immunosuppression resulting in increased infection, fluid retention, dyspnoea, graft-versus-host disease (GVHD; following allo-SCT) and organ toxicity (interstitial pneumonitis, veno-occlusive disease)
- additional supportive care required to address the immunosuppressive effects and long-term side effects of therapy for patients treated with allo-SCT – issues may include infertility, GVHD, increased risk of infection, anaemia, bleeding, mouth ulcers and fatigue
- early recognition and prompt initiation of corticosteroids for differentiation syndrome (NCCN 2015) and consideration for interruption of therapy when required
- chemically induced menopause that leads to atrophic vaginitis and dyspareunia, and changes in androgens that may alter libido and orgasm – these require sensitive discussion
- gastrointestinal symptoms, such as nausea, vomiting, severe mucositis, loss of appetite, dysgeusia, diarrhoea or constipation, as a result of treatment require optimal symptom control (with medication, total parenteral nutrition, analgesia and mouth care) and referral to a dietitian if dietary intake is affected
- malnutrition, which can occur as a result of disease or treatment (validated malnutrition screening tools should be used at the key points in the care pathway to identify patients at risk of malnutrition and refer to a dietitian for nutrition intervention)
- cognitive impairment, which patients treated with allo-SCT report to be a major component of quality-of-life impairment and can last for years post procedure (Buchbinder et al. 2018)
- assistance with managing complex medication regimens, multiple medications, assessment of side effects and assistance with difficulties swallowing medications (referral to a pharmacist may be required)
- decline in mobility or functional status as a result of treatment
- assistance with beginning or resuming regular exercise with referral to an exercise physiologist or physiotherapist (COSA 2018; Hayes et al. 2019).
Early involvement of general practitioners may lead to improved cancer survivorship care following acute treatment. General practitioners can address many supportive care needs through good communication and clear guidance from the specialist team (Emery 2014).
Patients, carers and families may have these additional issues and needs:
- financial issues related to loss of income (through reduced capacity to work or loss of work) and additional expenses as a result of illness or treatment
- advance care planning, which may involve appointing a substitute decision-maker and completing an advance care directive
- legal issues (completing a will, care of dependent children) or making an insurance, superannuation or social security claim on the basis of terminal illness or permanent disability.
Cancer Council’s 13 11 20 information and support line can assist with information and referral to local support services.
For more information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
The term ‘cancer survivor’ describes a person living with cancer, from the point of diagnosis until the end of life. Survivorship care in Australia has traditionally been provided to patients who have completed active treatment and are in the post-treatment phase. But there is now a shift to provide survivorship care and services from the point of diagnosis to improve cancer-related outcomes.
Cancer survivors may experience inferior quality of life and cancer-related symptoms for up to five years after their diagnosis (Jefford et al. 2017). Distress, fear of cancer recurrence, fatigue, obesity and sedentary lifestyle are common symptoms reported by cancer survivors (Vardy et al. 2019).
In the past two decades, the number of people surviving AML has increased. Approximately 60–70 per cent of AML patients under 60 years of age who receive intensive chemotherapy can expect to attain complete remission. More than 25 per cent of adults with AML (about 45 per cent of those who attain complete remission) can be expected to survive three or more years and may be cured.
International research shows there is an important need to focus on helping cancer survivors cope with life beyond their acute treatment. Cancer survivors often face issues that are different from those experienced during active treatment for cancer and may include a range of issues, as well as unmet needs that affect their quality of life (Lisy et al. 2019; Tan et al. 2019).
Physical, emotional and psychological issues include fear of cancer recurrence, cancer-related fatigue, pain, distress, anxiety, depression, cognitive changes and sleep issues (Lisy et al. 2019). Late effects may occur months or years later and depend on the type of cancer treatment. Survivors and their carers may experience impacted relationships and practical issues including difficulties with return to work or study and financial hardship. They may also experience changes to sex and intimacy. Fertility, contraception and pregnancy care after treatment may require specialist input.
The Institute of Medicine, in its report From cancer patient to cancer survivor: Lost in transition, describes the essential components of survivorship care listed in the paragraph above, including interventions and surveillance mechanisms to manage the issues a cancer survivor may face (Hewitt et al. 2006). Access to a range of health professions may be required including physiotherapy, occupational therapy, social work, dietetics, clinical psychology, fertility and palliative care. Coordinating care between all providers is essential to ensure the patient’s needs are met.
Cancer survivors are more likely than the general population to have and/or develop comorbidities (Vijayvergia & Denlinger 2015). Health professionals should support survivors to self-manage their own health needs and to make informed decisions about lifestyle behaviours that promote wellness and improve their quality of life (Australian Cancer Survivorship Centre 2016; Cancer Australia 2017; NCSI 2015).
Units treating AML should seek to develop specialised survivorship programs for patients who have completed anti-leukaemia therapy or consider referral to services where this is available.
The transition from active treatment to post-treatment care is critical to long-term health. In some cases, people will need ongoing, hospital-based care, and in other cases a shared follow-up care arrangement with their general practitioner may be appropriate. This will vary depending on the type and stage of cancer and needs to be planned.
Shared follow-up care involves the joint participation of specialists and general practitioners in the planned delivery of follow-up and survivorship care. A shared care plan is developed that outlines the responsibilities of members of the care team, the follow-up schedule, triggers for review, plans for rapid access into each setting and agreement regarding format, frequency and triggers for communication.
After completing initial treatment, a designated member of the multidisciplinary team (most commonly nursing or medical staff involved in the patient’s care) should provide the patient with a needs assessment and treatment summary and develop a survivorship care plan in conjunction with the patient. This should include a comprehensive list of issues identified by all members of the multidisciplinary team involved in the patient’s care and by the patient. These documents are key resources for the patient and their healthcare providers and can be used to improve communication and care coordination.
The treatment summary should cover, but is not limited to:
- the diagnostic tests performed and results
- diagnosis including stage, prognostic or severity score
- treatment received (types and dates)
- current toxicities (severity, management and expected outcomes)
- interventions and treatment plans from other health providers
- potential long-term and late effects of treatment
- supportive care services provided
- follow-up schedule
- contact information for key healthcare providers.
Responsibility for follow-up care should be agreed between the lead clinician, the general practitioner, relevant members of the multidisciplinary team and the patient. This is based on guideline recommendations for post-treatment care, as well as the patient’s current and anticipated physical and emotional needs and preferences.
Evidence comparing shared follow-up care and specialised care indicates equivalence in outcomes including recurrence rate, cancer survival and quality of life (Cancer Research in Primary Care 2016).
Ongoing communication between healthcare providers involved in care and a clear understanding of roles and responsibilities is key to effective survivorship care.
In particular circumstances, other models of post-treatment care can be safely and effectively provided such as nurse-led models of care (Monterosso et al. 2019). Other models of post-treatment care can be provided in these locations or by these health professionals:
- in a shared care setting
- in a general practice setting
- by non-medical staff
- by allied health or nurses
- in a non-face-to-face setting (e.g. by telehealth).
A designated member of the team should document the agreed survivorship care plan. The survivorship care plan should support wellness and have a strong emphasis on healthy lifestyle changes such as a balanced diet, a non-sedentary lifestyle, weight management and a mix of aerobic and resistance exercise (COSA 2018; Hayes et al. 2019).
This survivorship care plan should also cover, but is not limited to:
- what medical follow-up is required (surveillance for recurrence or secondary and metachronous cancers, screening and assessment for medical and psychosocial effects)
- model of post-treatment care, the health professional providing care and where it will be delivered
- care plans from other health providers to manage the consequences of cancer and cancer treatment
- wellbeing, primary and secondary prevention health recommendations that align with chronic disease management principles
- rehabilitation recommendations
- available support services
- a process for rapid re-entry to specialist medical services for suspected recurrence.
Survivors generally need regular follow-up, often for five or more years after cancer treatment finishes. For immediate post-therapy follow-up, the frequency of consultations will be determined by the patient’s needs and may range between several times a week and six-weekly. The primary treating clinical haematologist should coordinate these, with input from the full spectrum of allied health professionals. Follow-up frequency will usually reduce over time for patients in remission.
For longer term follow-up and surveillance of patients with AML, the frequency of disease assessment will be based on whether the patient is in remission or has relapsed/progressive disease.
The general surveillance schedule for patients in first remission is:
- for the first two to three years after treatment: full blood examination (FBE), and clinical assessment with a careful history and physical examination every three months
- thereafter, up to five years post-treatment: FBE and clinical review every three to six months
- then as deemed appropriate for individual patients: annual FBE and clinical review indefinitely.
For select patients undergoing intensive initial therapy, assessing for the presence or absence of MRD after consolidation therapy has been shown to predict later overt recurrence. This is also an area of very active research, and the evidence base is evolving rapidly. Currently, MRD assessment (+/– monitoring; see step 6) is appropriate for patients whose AML has one of the following molecular abnormalities: PML-RARA, CBFB-MYH11, RUNX1-RUNX1T1 or NPM1 mutation (Schuurhuis et al. 2018). Alternatively, flow cytometry for detecting phenotypically aberrant ‘different from normal’ populations in specialised laboratories may be considered.
Patients who have received allo-SCT will require specific long-term follow-up plans coordinated by the survivorship program at the transplant unit (Hilgendorf et al. 2015). In particular circumstances, follow-up care can be safely and effectively provided:
- in the general practice setting
- in the specialist and hospital setting, including in specialised late effects clinics staffed with members of the multidisciplinary team including physiotherapy, occupational therapy, nursing, social work, dietetics, clinical psychology and palliative care.
Processes for rapid re-entry to hospital care should be documented and communicated to the patient and relevant stakeholders.
Care in the post-treatment phase is driven by predicted risks (e.g. the risk of recurrence, developing late effects of treatment and psychological issues) as well as individual clinical and supportive care needs. It is important that post-treatment care is based on evidence and is consistent with guidelines. Not all people will require ongoing tests or clinical review and may be discharged to general practice follow-up.
The lead clinician should discuss (and general practitioner reinforce) options for follow-up at the start and end of treatment. It is critical for optimal aftercare that the designated member of the treatment team educates the patient about the symptoms of recurrence.
General practitioners (including nurses) can:
- connect patients to local community services and programs
- manage long-term and late effects
- manage comorbidities
- provide wellbeing information and advice to promote self-management
- screen for cancer and non-cancerous conditions.
Templates and other resources to help with developing treatment summaries and survivorship care plans are available from these organisations:
- Australian Cancer Survivorship Centre
- Cancer Australia – Principles of Cancer Survivorship
- Cancer Council Australia and states and territories
- Clinical Oncology Society of Australia – Model of Survivorship Care
- eviQ – Cancer survivorship: introductory course
- MyCarePlan.org.au
- South Australian Cancer Service – Statewide Survivorship Framework resources
- American Society of Clinical Oncology – guidelines.
Not smoking, eating a healthy diet, being sun smart, avoiding or limiting alcohol intake, being physically active and maintaining a healthy body weight may help reduce the risk of primary recurrence or a second primary cancer.
Encourage and support all cancer survivors to reduce modifiable risk factors for recurrence as well as other chronic diseases. Ongoing coordination of care between providers should also deal with any comorbidities, particularly ongoing complex and life-threatening comorbid conditions.
Participation in clinical trials, registries and tissue banking, where available, is considered a standard of care for patients with AML. Cross-referral between clinical trials centres should be encouraged to facilitate participation.
For more information visit the Cancer Australia website.
See validated screening tools mentioned in Principle 4 ‘Supportive care’. Additionally, the ‘Cancer Survivors Unmet Needs (CaSun)’ is another validated screening tool that may help health professionals to identify the unmet needs of patients during survivorship.
A number of specific challenges and needs may arise for cancer survivors:
- endocrine effects (gonadal), cardiac effects, osteoporosis, transfusional iron overload and secondary myelodysplasia in the late stages of therapy
- malnutrition due to ongoing treatment side effects, such as gastrointestinal symptoms, reduced appetite and reduced oral intake; this requires monitoring and nutrition intervention where indicated
- financial and employment issues (such as loss of income and assistance with returning to work, and the cost of treatment, travel and accommodation)
- appointing a substitute decision-maker and completing an advance care directive
- legal issues such as completing a will.
For more information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
Rehabilitation may be required at any point of the care pathway from the pre-treatment phase through to disease-free survival and palliative care (Cormie et al. 2017).
Issues that may need to be dealt with include managing cancer-related fatigue, coping with cognitive changes, improving physical endurance, achieving independence in daily tasks, returning to study or work and ongoing adjustment to cancer and its sequels.
Exercise is a safe and effective intervention that improves the physical and emotional health and wellbeing of cancer patients. Exercise should be embedded as part of standard practice in cancer care and be viewed as an adjunct therapy that helps counteract the adverse effects of cancer and its treatment.
Cancer survivors may find referral to specific cancer rehabilitation, optimisation programs or community-based rehabilitation appropriate and beneficial. Other options include referral to allied health supports through team care arrangements and mental health plans. Some community support organisations (cancer-related non-government, not-for-profit and charities) provide services to cancer survivors.
The lead clinician (themselves or by delegation) should take responsibility for these tasks:
- explaining the model of post-treatment care and the roles of health professionals involved in post-treatment care including the role of general practice
- explaining the treatment summary and follow-up care plan
- discussing the development of a shared follow-up and survivorship care plan where a model of shared follow-up care has been agreed
- discussing how to manage any of the physical, psychological or emotional issues identified
- providing information on the signs and symptoms of recurrent disease
- providing a survivorship care plan with information on secondary prevention and healthy living
- providing contact details of the care team involved
- providing clear information about the role and benefits of palliative care and advance care planning.
The lead clinician should ensure regular, timely, two-way communication with the general practitioner about:
- the patient’s progress
- the follow-up care plan
- potential late effects
- supportive and palliative care requirements
- any shared care arrangements
- clarification of various roles in patient care
- a process for rapid re-entry to medical services for patients with suspected recurrence or if there are other concerns.
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
Patients who present with relapsed or refractory disease should be managed by a multidisciplinary team and offered timely referral to appropriate physical, practical and emotional support.
The likelihood of relapsed or refractory disease is increased in patients who cannot tolerate standard consolidation treatment and in patients with intermediate/adverse risk factors not proceeding to transplantation.
Supportive care is integral to the care of all patients with relapsed or refractory AML.
For patients suitable for active anti-leukaemia therapy, repeat assessment of relevant genomic abnormalities is appropriate, even if no targetable lesions were identified in the initial diagnostic assessment.
Where patients have exhausted specific anti-leukaemic therapies or are intolerant of further anti-leukaemic therapy, symptomatic management is preferred, with the priority focused on quality of life.
Relapse occurs in more than 50 per cent of patients, and treatment outcomes will vary depending on individual prognostic factors. Most cases of relapsed AML are diagnosed through routine follow-up or by the patient presenting with symptoms.
Molecular monitoring for MRD is recommended for patients with APL, CBF leukaemia and NPM1 mutant AML (Döhner et al. 2010, 2017; Sanz et al. 2019). Comprehensive guidelines about the performance, monitoring frequency and interpretation of MRD technologies (flow cytometry and molecular) are available (Schuurhuis et al. 2018).
In general, bone marrow sampling is more sensitive than peripheral blood monitoring. The optimal frequency and duration of testing continues to be refined, with current guidelines provided by Schuurhuis et al. (2018).
If relapse is suspected, investigations should include:
- full blood count with blood film examination
- bone marrow aspiration and trephine, including flow cytometry, cytogenetic analysis and molecular testing (depending on clinical context).
Managing relapsed or refractory disease is complex and should therefore involve all the appropriate specialties in a multidisciplinary team including palliative care where appropriate. From the time of diagnosis, the team should offer patients appropriate psychosocial care, supportive care, advance care planning and symptom-related interventions as part of their routine care. The approach should be personalised to meet the patient’s individual needs, values and preferences. The full complement of supportive care measures as described throughout the optimal care pathway and in Appendices A and B, and in the special population groups section should be offered to assist patients and their families and carers to cope. These measures should be updated as the patient’s circumstances change.
Access to the best available therapies, including clinical trials are crucial to achieving the best outcomes for anyone with relapsed or refractory disease. Novel therapies are becoming available for patients with relapsing and refractory disease. Identification of relevant targets may require repeat molecular assessment to identify lesions newly emergent at the time of treatment failure.
Survivorship care should be considered and offered at an early stage. Many people live with advanced cancer for many months or years. As survival is improving in many patients, survivorship issues should be considered as part of routine care. Health professionals should therefore be ready to change and adapt treatment strategies according to disease status, prior treatment tolerance and toxicities and the patient’s quality of life, in addition to the patient’s priorities and life plans.
If there is an indication that a patient’s cancer has returned, care should be provided under the guidance of a treating specialist. Each patient should be evaluated to determine if referral to the original multidisciplinary team is necessary. Often referral back to the original multidisciplinary team will not be necessary unless there are obvious aspects of care involving different therapeutic and supportive care disciplines not otherwise accessible. The multidisciplinary team may include new members such as palliative care specialists.
The intent and nature of treatment will depend on the location, extent of relapsed or refractory disease, previous management, comorbidities and the patient’s preferences.
In managing people with relapsed AML, anti-leukaemia treatment may include these options:
- intensive re-induction – chances of success are better after a longer duration of the first remission and can be estimated using the European Prognostic Index (Breems et al. 2005)
- enrolment into a clinical trial – trials evaluating the use of novel agents should be strongly considered
- allo-SCT
- irradiation of infiltrative/mass lesions (solitary chloromas) and craniospinal irradiation in the event of central nervous system disease
- regular transfusional support with red cells and platelets should be administered where appropriate.
If treatment is given with curative intent, the facilities need to be of the same level as for the initial therapy. Palliative chemotherapy may be delivered in a less specialised environment. Allo-SCT must be delivered only in specialised units with appropriate accreditation as well as human and physical resources.
The potential goals of treatment should be discussed, respecting the patient’s cultural values. Wherever possible, written information should be provided.
Encourage early referral to clinical trials or accepting an invitation to participate in research.
Advance care planning is important for all patients with a cancer diagnosis but especially those with advanced disease. Patients should be encouraged to think and talk about their healthcare values and preferences with family or carers, appoint a substitute decision-maker and consider developing an advance care directive to convey their preferences for future health care in the event they become unable to communicate their wishes (AHMAC 2011).
Refer to section 4.4 ‘More information’ for links to resources.
Refer patients and carers to Advance Care Planning Australia or to the Advance Care Planning National Phone Advisory Service on 1300 208 582.
Early referral to palliative care can improve the quality of life for people with AML and in some cases may be associated with survival benefits (Haines 2011; Temel et al. 2010; Zimmermann et al. 2014). The treatment team should emphasise the value of palliative care in improving symptom management and quality of life to patients and their carers. Refer to section 4.4 for more detailed information.
The lead clinician should ensure timely and appropriate referral to palliative care services. Referral to palliative care services should be based on the patient’s need and potential for benefit, not prognosis.
Refer to the end of section 4.4 ‘Palliative care’ for links to resources.
Participation in clinical trials, registries and tissue banking, where available, is considered tandard of care for patients with AML. Cross-referral between clinical trials centres should be encouraged to facilitate participation.
For more information visit the Cancer Australia website.
See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific challenges and needs may arise at this time for patients:
- assistance for dealing with emotional and psychological distress resulting from fear of death or dying, existential concerns, anticipatory grief, communicating wishes to loved ones, interpersonal problems and sexuality concerns
- potential isolation from normal support networks, particularly for rural patients who are staying away from home for treatment
- cognitive changes as a result of treatment and disease progression such as altered memory, attention and concentration (a patient may appoint someone to make medical, financial and legal decisions on their behalf – a substitute decision-maker – before and in case they experience cognitive decline)
- side effects resulting from high-dose chemotherapy including alopecia, fatigue, damage to the bone marrow and other quickly growing tissues, immunosuppression, fluid retention, dyspnoea, GVHD, organ toxicity (interstitial pneumonitis, veno-occlusive disease), episodic hypotension and pulmonary infiltrates
- chemically induced menopause that leads to atrophic vaginitis and dyspareunia, and changes in androgens that may alter libido function and orgasm (these issues require sensitive discussion)
- differentiation syndrome – early recognition and prompt initiation of corticosteroids is required (NCCN 2019) and consideration given to interrupting therapy
- gastrointestinal symptoms such as nausea, vomiting, severe mucositis, loss of appetite, dysgeusia, diarrhoea or constipation as a result of treatment, which requires optimal symptom control with medicine, total parenteral nutrition, analgesia and mouth care (referral to a dietitian may be required if dietary intake is affected)
- decline in mobility or functional status as a result of recurrent disease and treatments (referral to physiotherapy or occupational therapy may be required)
- coping with hair loss and changes in physical appearance (refer to the Look Good, Feel Better program– see ’Resource List’)
- appointing a substitute decision-maker and completing an advance care directive
- financial issues as a result of disease recurrence such as gaining early access to superannuation and insurance
- legal issues (completing a will, care of dependent children) and making an insurance, superannuation or social security claim on the basis of terminal illness or permanent disability.
For patients treated with allo-SCT, additional supportive care may be required to address the immunosuppressive and long-term side effects of therapy. Issues may include infertility, GVHD, increased risk of infection, anaemia, bleeding, mouth ulcers and fatigue.
Rehabilitation may be required at any point of the metastatic care pathway, from preparing for treatment through to palliative care. Issues that may need to be dealt with include managing cancer-related fatigue, improving physical endurance, achieving independence in daily tasks, returning to work and ongoing adjustment to cancer and its sequels.
Exercise is a safe and effective intervention that improves the physical and emotional health and wellbeing of cancer patients. Exercise should be embedded as part of standard practice in cancer care and be viewed as an adjunct therapy that helps counteract the adverse effects of cancer and its treatment.
The lead clinician should ensure there is adequate discussion with patients and carers about the diagnosis and recommended treatment, including treatment intent and possible outcomes, likely adverse effects and the supportive care options available.
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
Step 7 is concerned with maintaining the patient’s quality of life and meeting their health and supportive care needs as they approach the end of life, as well as the needs of their family and carers.
Some patients with advanced cancer will reach a time when active treatment is no longer appropriate. The team needs to share the principles of a palliative approach to care when making decisions with the patient and their family or carer. End-of-life care is appropriate when the patient’s symptoms are increasing and functional status is declining.
If the treatment team does not include a palliative care member, the lead clinician should consider referring the patient to palliative care services, with the general practitioner’s engagement. This may include inpatient palliative unit access (as required).
The multidisciplinary team may consider seeking additional expertise from these professionals:
- clinical psychologist
- clinical nurse specialist or practitioner
- social worker
- palliative medicine specialist
- pain specialist
- pastoral or spiritual carer
- bereavement counsellor
- music therapist
- art therapist
- cultural expert
- Canteen for children of parents with cancer
The team might also recommend that patients access these services:
- home and community-based care
- specialist community palliative care workers
- community nursing.
If the patient does not already have an advance care directive in place, a designated member of the treatment team should encourage them to develop one in collaboration with their family or carer (AHMAC 2011).
It is essential for the treatment team to consider the appropriate place of care, the patient’s preferred place of death and the support needed for the patient, their family and carers.
The treatment team should also ensure that carers and families receive the information, support and guidance about their role according to their needs and wishes (Palliative Care Australia 2018).
The treatment team can refer patients and carers to these resources:
- Palliative Care Australia
- Advance Care Planning Australia or to Advance Care Planning Australia’s National Advisory Service on 1300 208 582.
Clinical trials may help improve palliative care and in managing a patient’s symptoms of advanced cancer (Cancer Council Victoria 2019). The treatment team should support the patient to participate in research and clinical trials where available and appropriate.
For more information visit the Cancer Australia website. See ’Resource list’ for additional clinical trial databases.
See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific challenges and needs may arise for patients at this time:
- assistance for dealing with emotional and psychological distress from anticipatory grief, fear of death or dying, anxiety/depression and interpersonal problems
- management of physical symptoms such as pain and fatigue, reduced appetite, early satiety and weight loss
- transfusion support, which requires specialised clinical transfusion knowledge
- decline in mobility or functional status, affecting the patient’s discharge destination (a referral to physiotherapy, exercise physiology, occupational therapy or social work may be needed)
- appointing a substitute decision-maker and completing an advance care directive
- legal issues (completing a will, care of dependent children) and making an insurance, superannuation or social security claim on the basis of terminal illness or permanent disability
- specific support for families where a parent is dying and will leave behind bereaved children or adolescents, creating special family needs
- arranging a funeral.
These services and resources can help:
- referral to 13 11 20 for Cancer Council Australia’s Pro Bono Program for free legal, financial, small business accounting and workplace assistance (subject to a means test)
- Sad news sorry business (Queensland Health 2015) for the specific needs of Aboriginal and Torres Strait Islander people.
For more information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
The lead clinician is responsible for:
- being open to and encouraging discussion with the patient about the expected disease course, considering the patient’s personal and cultural beliefs and expectations
- discussing palliative care options, including inpatient and community-based services as well as dying at home and subsequent arrangements
- providing the patient and carer with the contact details of a palliative care service
- referring the patient to palliative care in the community according to the carer’s wishes.
The lead clinician should discuss end-of-life care planning to ensure the patient’s needs and goals are met in the appropriate environment. The patient’s general practitioner should be kept fully informed and involved in major developments in the patient’s illness path.
For support with communication skills and training programs, see these sources:
The burden of cancer is not evenly spread across Australia. People experiencing socioeconomic disadvantage, Aboriginal and Torres Strait Islander communities, culturally diverse communities, people living with a disability, people with chronic mental health or psychiatric concerns and those who live in regional and rural areas of Australia have poorer cancer outcomes.
Cancer is the third leading cause of burden of disease for Aboriginal and Torres Strait Islander people. While Australia’s cancer survival rates are among the best in the world, Aboriginal and Torres Strait Islander people continue to experience a different pattern of cancer incidence and significant disparities in cancer outcomes compared with non-Indigenous Australians.
For Aboriginal and Torres Strait Islander people, health and connection to land, culture, community and identity are intrinsically linked. Health encompasses a whole-of-life view and includes a cyclical concept of life–death–life.
The distinct epidemiology of cancer among Aboriginal and Torres Strait Islander people, and unique connection to culture, highlight the need for a specific optimal care pathway for Aboriginal and Torres Strait Islander people with cancer. Ensuring this pathway is culturally safe and supportive is vital to tackling the disparities for Aboriginal and Torres Strait Islander people.
Published in 2018, the Optimal care pathway for Aboriginal and Torres Strait Islander people with cancer provides guidance to health practitioners and service planners on optimal care for Aboriginal and Torres Strait Islander people with cancer across the cancer continuum.
In addition to the key principles underpinning cancer-specific pathways, these are the key concepts that are fundamental to Aboriginal and Torres Strait Islander health:
- providing a holistic approach to health and wellbeing
- providing a culturally appropriate and culturally safe service
- acknowledging the diversity of Aboriginal and Torres Strait Islander peoples
- understanding the social determinants and cultural determinants of health (Cancer Australia 2015).
To view the Optimal care pathway for Aboriginal and Torres Strait Islander people with cancer, visit the Cancer Australia website. To view the consumer resources – Checking for cancer and Cancer, visit the Cancer Australia website.
For people from culturally diverse backgrounds in Australia, a cancer diagnosis can come with additional complexities, particularly when English proficiency is poor. In many languages there is not a direct translation of the word ‘cancer’, which can make communicating vital information difficult. Perceptions of cancer and related issues can differ greatly in people from culturally diverse backgrounds and this can affect their understanding and decision making after a cancer diagnosis. In addition to different cultural beliefs, when English language is limited there is potential for miscommunication of important information and advice, which can lead to increased stress and anxiety for patients.
A professionally trained interpreter (not a family member or friend) should be made available when communicating with people with limited English proficiency. Navigation of the Australian healthcare system can pose problems for those with a non-Anglo culture, and members of the treatment teams should pay particular attention to supporting these patients.
The Australian Cancer Survivorship Centre has developed a glossary of more than 700 cancer terms in nine different languages. The multilingual glossary has been designed as a resource for professional translators, interpreters and bilingual health professionals working in the cancer field. The glossary is a unique tool that enables language professionals with access to accurate, consistent and culturally appropriate terminology.
Visit the Peter Mac website to see the glossary.
Disability, which can be physical, intellectual or psychological, may have existed before the cancer diagnosis or may be new in onset (occurring due to the cancer treatment or incidentally). Adjusting to life with a disability adds another challenge to cancer care and survivorship.
Several barriers prevent people with disabilities from accessing timely and effective health care (AIHW 2017):
- physical limitations
- competing health needs
- the trauma of undergoing invasive procedures
- potential barriers associated with obtaining informed consent
- failure to provide assistance with communication
- lack of information
- discriminatory attitudes among healthcare staff.
In caring for people with disabilities and a cancer diagnosis, the Australian Institute of Health and Welfare disability flag should be used at the point of admittance to correctly identify and meet the additional requirements of a person with disability. Facilities should actively consider access requirements, and health practitioners should make reasonable adjustments where required.
Patients aged between seven and 65 years who have a permanent or significant disability may be eligible for support or funding through the National Disability Insurance Scheme (National Disability Insurance Agency 2018). More information can be found on the NDIS website.
Patients aged 65 years or older (50 years or older for Aboriginal or Torres Strait Islander people) may be eligible for subsidised support and services through aged care services. An application to determine eligibility can be completed online over the phone. More information can be found at the My Aged Care website.
‘Talking End of Life’ is a resource that shows how to teach people with intellectual disability about end of life. It is designed for disability support workers but is also helpful for others including families, health professionals and educators.
To view the resource, visit the Talking End of Life website.
The remission rate in adult AML patients is inversely related to age. Data suggests that once attained, duration of remission may be shorter in older patients (NCI 2020). Older patients are more likely to have drug-resistant disease, frequently have major comorbidities and are less able to tolerate intensive chemotherapy.
Planning and delivering appropriate cancer care for older people can present a number of challenges. This could also be true for frail people or those experiencing comorbidities. Effective communication between oncology and geriatrics departments will help facilitate best practice care, which takes into account physiological age, complex comorbidities, risk of adverse events and drug interactions, as well as the implications of cognitive impairment on suitability of treatment and consent (Steer et al. 2009).
At a national interdisciplinary workshop convened by the Clinical Oncology Society of Australia, it was recommended that people over the age of 70 undergo some form of geriatric assessment, in line with international guidelines (COSA 2013; palliAGED 2018). Screening tools can be used to identify those patients in need of a comprehensive geriatric assessment (Decoster et al. 2015). This assessment can be used to help determine life expectancy and treatment tolerance and guide appropriate referral for multidisciplinary intervention that may improve outcomes (Wildiers et al. 2014).
Frailty is not captured through traditional measures of performance status (e.g. ECOG) and includes assessment in the domains of:
- function
- comorbidity
- presence of geriatric syndromes
- nutrition
- polypharmacy
- cognition
- emotional status
- social supports.
Despite very intensive therapeutic options, paediatric patients diagnosed with AML still have a relatively poor prognosis in comparison with many paediatric or adolescent and young adult patients with another cancer diagnosis (including acute lymphoblastic leukaemia). Toxicity from current regimens leaves little room for further intensification to improve outcomes. The side effects of intensive systemic therapy can be even more severe for children and include acute organ toxicities, prolonged immunodeficiency and infection. As a result of these complexities, high-quality evidence-based care is required not only to deliver therapy and supportive care but is essential in the diagnosis phase, post-treatment surveillance and long-term follow-up care.
Children with AML must have their treatment delivered by a statewide referral centre for paediatric oncology. Shared care may be considered for surveillance after completing treatment. Children’s cancer services actively participate in clinical trials as a way of participating in research and improving outcomes for children.
In recent years, adolescent and young adult oncology has emerged as a distinct field due to lack of progress in survival and quality-of-life outcomes (Ferrari et al. 2010; Smith et al. 2013). The significant developmental change that occurs during this life stage complicates a diagnosis of cancer, often leading to unique physical, social and emotional effects for young people at the time of diagnosis and throughout the cancer journey (Smith et al. 2012).
In caring for young people with cancer, akin to the comorbidities that require specific care in the older cancer population, the treatment team needs to pay careful attention to promoting normal development (COSA 2014). This requires personalised assessments and management involving a multidisciplinary, disease-specific, developmentally targeted approach that adheres to the following principles:
- understanding the developmental stages of adolescence and supporting normal adolescent health and development alongside cancer management
- understanding and supporting the rights of young people
- communication skills and information delivery that are appropriate to the young person
- meeting the needs of all involved, including the young person, their carers and their family
- working with educational institutions and workplaces
- considering survivorship and palliative care needs.
An oncology team caring for an adolescent or young adult with cancer should be able to demonstrate these specific areas of expertise:
- be able to ensure access to expert adolescent and young adult health providers who have knowledge specific to the biomedical and psychosocial needs of the population
- understand the biology and current management of the disease in the adolescent and young adult age group
- consider participating in research and clinical trials for each patient
- engage in proactive discussion and management of fertility preservation, late effects of treatment, ongoing need for contraception, and psychosocial and psychosexual needs
- provide treatment in an environment that is friendly to adolescents and young adults.
In general, people from lower socioeconomic groups are at greater risk of poor health, have higher rates of illness, disability and death, and live shorter lives than those from higher socioeconomic groups (AIHW 2016). People experiencing socioeconomic disadvantage are less likely to participate in screening programs, more likely to be obese, less likely to exercise and much more likely to smoke, which are all risk factors for cancer. In 2010–2014 age-standardised cancer incidence rates were higher in the lowest socioeconomic areas compared with the highest socioeconomic areas for all cancers combined (Cancer Australia 2019b).
Socioeconomic status and low health literacy are closely correlated. Therefore, effective communication with patients and carers is particularly important given the prevalence of low health literacy in Australia (estimated at 60 per cent of Australian adults) (ACSQHC 2014).
Consideration should be taken for cancer patients experiencing socioeconomic disadvantage to reduce their risk of being underserved for health care.
A diagnosis of cancer may present additional challenges to people who have pre-existing chronic mental health or psychiatric concerns, resulting in exacerbation of their mental health symptoms. This may include heightened anxiety, worsening depression or thoughts of self-harm.
As poor adjustment and coping can affect treatment decisions, people who are known to have a mental health diagnosis need psychosocial assessment in the oncology setting to formulate a plan for ongoing support throughout treatment.
Psychosocial support can assist with challenges in communicating with health professionals, enhance understanding of the treatment journey, ensure capacity for consent to treatment options and improve compliance with treatment requests. A referral for psychosocial support from a health professional to the psycho-oncology team can ensure these patients are provided with targeted interventions or referrals to community-based services that may mitigate problems associated with the impacts of social isolation that frequently accompany chronic mental ill-health.
Many patients with chronic mental health problems may be well known to external service providers. Psycho-oncology health professionals can form meaningful partnerships with existing service providers to optimise patient care throughout treatment and beyond.
Drug use disorders fall within the area of mental health conditions. People who are opiate dependent may have specific and individual requirements regarding pain management and their own preference for type of opiate prescribed or used.
People who identify as sexually or gender diverse may have unique needs following a cancer diagnosis. Sexually or gender diverse identities include (but are not limited to) people who identify as lesbian, gay, bisexual or transgender, collectively ‘LGBT’. There is no universally agreed upon initialism to describe this community, with other terms such as queer/questioning (Q), intersex (I), asexual (A) and pansexual (P) often included, as well as a plus symbol (+) indicating inclusivity of other identities not explicitly mentioned.
Sexual orientation and gender identity are relevant across the entire spectrum of cancer care, from prevention to survivorship and end-of-life care. LGBT people are less likely to participate in cancer screening, and some segments of the LGBT community exhibit elevated rates of specific cancer risk factors – for example, higher rates of smoking and alcohol use. Regarding treatment, there may be unique factors relevant to LGBT people that may affect decision making. Additionally, the LGBT population experiences higher rates of anxiety, depression and stressful life circumstances, and may be at risk of inferior psychosocial outcomes following a cancer diagnosis. LGBT people are also more likely to be estranged from their families of origin, and for older people, less likely to have adult children who may provide support and care.
Barriers to care for LGBT people include past negative interactions with healthcare systems, experiences or fear of discrimination and harassment in healthcare settings, assumptions of cisgender/heterosexual identity, lack of recognition or exclusion of same-sex partners from care, and a lack of relevant supportive care and information resources.
To provide safe and appropriate care for LGBT people with cancer, healthcare providers should:
- display environmental cues to show an inclusive and safe setting for LGBT patients
- avoid assumptions about the sexual orientation or gender identity of patients and their partners
- facilitate positive disclosure of sexual orientation or gender identity
- include same-sex/gender partners and families of choice in care
- be aware of relevant supportive care and information resources
- provide non-judgemental, patient-centred care.
Supportive care in cancer refers to the following five domains:
- the physical domain, which includes a wide range of physical symptoms that may be acute, relatively short lived or ongoing, requiring continuing interventions or rehabilitation
- the psychological domain, which includes a range of issues related to the patient’s mental health wellbeing and personal relationships
- the social domain, which includes a range of social and practical issues that will affect the patient, carer and family such as the need for emotional support, maintaining social networks and financial concerns
- the information domain, which includes access to information about cancer and its treatment, recovery and survivorship support services and the health system overall
- the spiritual domain, which focuses on the patient’s changing sense of self and challenges to their underlying beliefs and existential concerns (Palliative Care Victoria 2019).
Fitch’s (2000) model of supportive care recognises the variety and level of intervention required at each critical point as well as the need to be specific to the individual patient. The model targets the type and level of intervention required to meet patients’ supportive care needs.
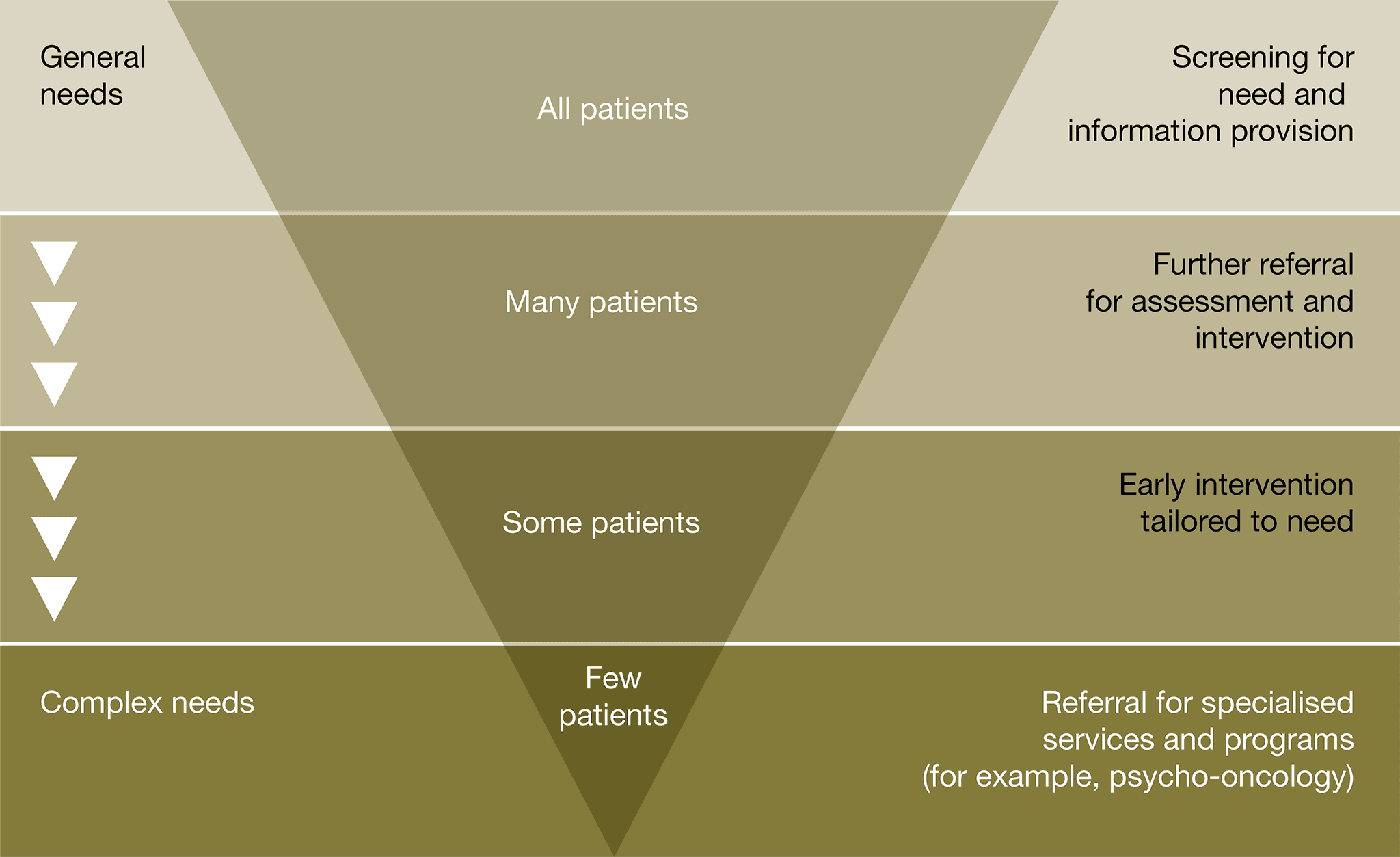
High-dose chemotherapy is both physically and emotionally demanding. People undergoing this treatment may feel exhausted, depressed or anxious for years after treatment (Jones et al. 2015). Regular screening and ongoing monitoring for depression is part of long-term follow-up.
Patients who have undergone a stem cell transplant may have cognitive impairments up to three years post procedure (Sharafeldin et al. 2018). Long-term follow-up and identification of strategies, such as maintaining written notes and repeating information, to enable patients to cope with alterations in cognitive function may be required.
Consider a referral to a psychologist, psychiatrist, pastoral/spiritual care practitioner, social worker, specialist nurse or a relevant community-based program if the patient has these issues:
- displaying emotional cues such as tearfulness, distress that requires specialist intervention, avoidance or withdrawal
- being preoccupied with or dwelling on thoughts about cancer and death
- displaying fears about the treatment process or the changed goals of their treatment
- displaying excessive fears about cancer progression or recurrence
- worrying about loss associated with their daily function, dependence on others and loss of dignity
- becoming isolated from family and friends and withdrawing from company and activities that they previously enjoyed
- feeling hopeless and helpless about the effect that cancer is having on their life and the disruption to their life plans
- struggling to communicate with family and loved ones about the implications of their cancer diagnosis and treatment
- experiencing changes in sexual intimacy, libido and function
- struggling with the diagnosis of metastatic or advanced disease
- having difficulties quitting smoking (refer to Quitline on 13 7848) or with other drug and alcohol use
- having difficulties transitioning to palliative care.
Additional considerations that may arise for the multidisciplinary team include:
- support for the carer – encourage referrals to psychosocial support from a social worker, psychologist or general practitioner
- referral to an exercise physiologist or physiotherapist as a therapeutic approach to prevent and manage psychological health
- referral to wellness-after-cancer programs to provide support, information and offer strategies.
Complementary therapies may be used together with conventional medical treatments to support and enhance quality of life and wellbeing. They do not aim to cure the patient’s cancer. Instead, they are used to help control symptoms such as pain and fatigue (Cancer Council Australia 2019).
The lead clinician or health professional involved in the patient’s care should discuss the patient’s use (or intended use) of complementary therapies not prescribed by the multidisciplinary team to assess safety and efficacy and to identify any potential toxicity or drug interactions.
The lead clinician should seek a comprehensive list of all complementary and alternative medicines being taken and explore the patient’s reason for using these therapies and the evidence base. A transparent and honest discussion that is free from judgement should be encouraged.
While some complementary therapies are supported by strong evidence, others are not. For such therapies, the lead clinician should discuss their potential benefits and use them alongside conventional therapies (NHMRC 2014).
If the patient expresses an interest in using complementary therapies, the lead clinician should consider referring patients to health providers within the multidisciplinary team who have expertise in the field of complementary and alternative therapies (e.g. a clinical pharmacist, dietitian or psychologist) to assist them to reach an informed decision. Costs of such approaches should be part of the discussion with the patient and considered in the context of evidence of benefit.
The lead clinician should assure patients who use complementary therapies that they can still access a multidisciplinary team review and encourage full disclosure about therapies being used.
- See Cancer Australia’s position statement on complementary and alternative therapies
- See the Clinical Oncological Society of Australia’s position statement Use of complementary and alternative medicine by cancer patients
Advance Care Planning Australia
Advance Care Planning Australia provides national advance care planning resources for individuals, families, health professional and service providers. Resources include a national advisory service, information resources, a legal forms hub and education modules.
- Telephone: 1300 208 582
- Website
Australasian Leukaemia and Lymphoma Group
The Australasian Leukaemia and Lymphoma Group (ALLG) is a not-for-profit clinical trial organisation that sponsors local and international investigator-initiated clinical trials. ALLG has a list of clinical trials available for patients with haematological malignancies including leukaemia and lymphoma.
Australian Cancer Survivorship Centre
The Australian Cancer Survivorship Centre has developed information resources and events to help people move from initial treatment to post treatment and beyond, including those receiving maintenance treatments. While they do not provide clinical advice, they connect with a range of providers to enable improved care.
- Telephone: (03) 8559 6220
- Website
Australian Commission on Safety and Quality in Health Care
The Australian Commission on Safety and Quality in Health Care has developed a resource for patients and carers explaining the coordination of care that patients should receive from their health service during cancer treatment. The resource is called What to expect when receiving medication for cancer care.
Beyond Blue
Beyond Blue provides information about depression, anxiety and related disorders, as well as about available treatment and support services.
- Telephone: 1300 22 4636
- Website
Cancer Australia
Cancer Australia provides information for consumers, carers and their families including printed resources and video content.
Cancer Council’s Cancer Information and Support Service
Cancer Council 13 11 20 is a confidential telephone support service available to anyone affected by cancer. This service acts as a gateway to evidence-based documented, practical and emotional support available through Cancer Council services and other community organisations. Calls will be answered by a nurse or other oncology professional who can provide information relevant to a patient’s or carer’s situation. Health professionals can also access this service.
- Telephone: 13 11 20 – Monday to Friday, 9.00am to 5.00pm (some states have extended hours)
- Website
Cancer Council’s Cancer Connect
Cancer Connect is a free and confidential telephone peer support service that connects someone who has cancer with a specially trained volunteer who has had a similar cancer experience.
A Connect volunteer can listen with understanding and share their experiences and ways of coping. They can provide practical information, emotional support and hope. Many people newly diagnosed with cancer find this one-to-one support very beneficial.
For more information on Cancer Connect call Cancer Council on 13 11 20.
Canteen
Canteen helps adolescents, young adults and parents to cope with cancer in their family. Canteen offers individual support services, peer support services and a youth cancer service, as well as books, resources and useful links.
- Telephone: 1800 835 932 to talk to a health professional about information and support for young people or 1800 226 833 for other enquiries
- Website
Clinical trial information
For a collection of clinical trials available in Australia see the following sources of information:
CanEAT pathway
A guide to optimal cancer nutrition for people with cancer, carers and health professionals.
Guides to best cancer care
The short guides help patients, carers and families understand the optimal cancer care that should be provided at each step. They include optimal timeframes within which tests or procedures should be completed, prompt lists to support patients to understand what might happen at each step of their cancer journey and to consider what questions to ask, and provide information to help patients and carers communicate with health professionals.
The guides are located on an interactive web portal, with downloadable PDFs available in multiple languages.
Leukaemia Foundation
Provides information, education and support programs for people living with lymphoma.
- Telephone: 1800 620 420 (Monday to Friday, 9.00am – 5.00pm)
- Website
Look Good, Feel Better
A free national community service program, run by the Cancer Patients Foundation, dedicated to teaching cancer patients how to manage the appearance-related side effects caused by treatment for any type of cancer.
- Telephone: 1800 650 960
- Website
Quitline
Quitline is a confidential, evidence-based telephone counselling service. Highly trained Quitline counsellors use behaviour change techniques and motivational interviewing over multiple calls to help people plan, make and sustain a quit attempt.
Quitline is a culturally inclusive service for all, and Aboriginal counsellors are also available. Health professionals can refer patients to Quitline online or via fax.
- Telephone: 13 7848
- Website or the relevant website in your state or territory.
Australasian Leukaemia and Lymphoma Group
The Australasian Leukaemia and Lymphoma Group (ALLG) is a not-for-profit clinical trial organisation that sponsors local and international investigator-initiated clinical trials. ALLG has a list of clinical trials available for patients with haematological malignancies including leukaemia and lymphoma.
Australian Cancer Survivorship Centre
The Australian Cancer Survivorship Centre provides expertise in survivorship care, information, support and education. Its purpose is to support and enable optimal survivorship care.
- Telephone: (03) 8559 6220
- Website
Australian Commission on Safety and Quality in Health Care
The Australian Commission on Safety and Quality in Health Care has developed a guide for clinicians containing evidence-based strategies to support clinicians to understand and fulfil their responsibilities to cancer patients. This guide is particularly relevant to steps 3 to 6 of the optimal care pathway. The guide is titled NSQHS Standards user guide for medication management in cancer care for clinicians.
Cancer Australia
Information for health providers including guidelines, cancer learnings, cancer guides, reports, resources, videos, posters and pamphlets.
Cancer Council Australia
Information on prevention, research, treatment and support provided by Australia’s peak independent cancer authority.
CanEAT pathway
A guide to optimal cancer nutrition for people with cancer, carers and health professionals.
eviQ
A clinical information resource providing health professionals with current evidence-based, peer-maintained, best practice cancer treatment protocols and information relevant to the Australian clinical environment.
Haematology Society of Australia and New Zealand
Provides support and advocacy for research and education in haematology, as well as opportunities for haematologists, scientists, nurses and students to promote scientific communication and education in the field of haematology.
National Health and Medical Research Council
Information on clinical practice guidelines, cancer prevention and treatment.
Advance care directive – voluntary person-led document that focus on an individual’s values and preferences for future health and medical treatment decisions, preferred outcomes and care. They are completed and signed by a competent person. They are recognised by specific legislation (statutory) or common law (non-statutory). Advance care directives can also appoint the substitute decision-maker(s) who can make decisions about health or personal care on the individual’s behalf if they are no longer able to make decisions themselves. Advance care directives focus on the future health care of a person, not on the management of his or her assets. They come into effect when an individual loses decision-making capacity.
Advance care planning – the process of planning for future health and personal care, where the person’s values, beliefs and preferences are made known so they can guide decision making at a future time when that person cannot make or communicate their decisions.
Alternative therapies – treatments used in place of conventional medical treatment.
Care coordinator – the health provider nominated by the multidisciplinary team to coordinate patient care. The care coordinator may change over time depending on the patient’s stage in the care pathway and the location and care in which care is being delivered.
Castrate resistant progressive disease – progressive disease despite castrate levels of testosterone (Cancer Council Australia Advanced Prostate Cancer Guidelines Working Party 2010).
Complementary therapies – supportive treatment used in conjunction with conventional medical treatment. These treatments may improve wellbeing and quality of life and help people deal with the side effects of cancer.
End-of-life care – includes physical, spiritual and psychosocial assessment, and care and treatment, delivered by health professionals and ancillary staff. It also includes support of families and carers and care of the patient’s body after their death.
Immunotherapy – a type of cancer treatment that helps the body’s immune system to fight cancer. Immunotherapy can boost the immune system to work better against cancer or remove barriers to the immune system attacking the cancer.
Indicator – a documentable or measurable piece of information regarding a recommendation in the optimal care pathway.
Informed financial consent – the provision of cost information to patients, including notification of likely out-of-pocket expenses (gaps), by all relevant service providers, preferably in writing, before admission to hospital or treatment (Commonwealth Department of Health 2017).
Lead clinician – the clinician who is nominated as being responsible for individual patient care. The lead clinician may change over time depending on the stage of the care pathway and where care is being provided.
Metastatic disease – cancer that has spread from the part of the body where it started (the primary site) to other parts of the body.
Multidisciplinary care – an integrated team approach to health care in which medical and allied health providers consider all relevant treatment options and collaboratively develop an individual treatment plan for each patient.
Multidisciplinary team – comprises the core disciplines that are integral to providing good care. The team is flexible in approach, reflects the patient’s clinical and psychosocial needs and has processes to facilitate good communication.
Multidisciplinary team meeting – a meeting of health professionals from one or more clinical disciplines who together make decisions about recommended treatment of patients.
Optimal care pathway – the key principles and practices required at each stage of the care pathway to guide the delivery of consistent, safe, high-quality and evidence-based care for all people affected by cancer.
Palliative care – any form of medical care or treatment that concentrates on reducing the severity of disease symptoms.
Patient management frameworks – tumour stream models adopted in Victoria in 2003 to reduce variation in cancer care. The optimal care pathways are updated versions of these models.
Performance status – an objective measure of how well a patient can carry out activities of daily life.
Prehabilitation – one or more interventions performed in a newly diagnosed cancer patient that are designed to improve physical and mental health outcomes as the patient undergoes treatment and beyond.
Primary care health professional – in most cases this is a general practitioner but may also include general practice nurses, community nurses, nurse practitioners, allied health professionals, midwives, pharmacists, dentists and Aboriginal health workers.
Primary specialist – the person who makes the referral to the multidisciplinary team (such as specialist physician, surgeon, oncologist, palliative care specialist). This person will also make referrals for treatment and will be responsible for overseeing follow-up care.
PSMA PET/CT – a whole body scan that images prostate cancer wherever it is located in the body.
Rehabilitation – comprises multidisciplinary efforts to allow the patient to achieve optimal physical, social, physiological and vocational functioning within the limits imposed by the disease and its treatment.
Spiritual care – the aspect of humanity that refers to the way individuals seek and express meaning and purpose and the way they experience their connectedness to the moment, to self, to others, to nature, and to the significant or sacred.
Substitute decision-maker – a person permitted under the law to make decisions on behalf of someone who does not have competence or capacity.
Supportive care – care and support that aims to improve the quality of life of people living with cancer, cancer survivors and their family and carers and particular forms of care that supplement clinical treatment modalities.
Survivorship – an individual is considered a cancer survivor from the time of diagnosis, and throughout their life; the term includes individuals receiving initial or maintenance treatment, in recovery or in the post-treatment phase.
Survivorship care plan – a formal, written document that provides details of a person’s cancer diagnosis and treatment, potential late and long-term effects arising from the cancer and its treatment, recommended follow-up, surveillance, and strategies to remain well.
Targeted therapy – a medicine that blocks the growth and spread of cancer by interfering with specific molecules.
We acknowledge the Traditional Owners of Country throughout Australia and their continuing connection to the land, sea and community. We pay our respects to them and their cultures and to Elders past, present and emerging.
This work is available from the Cancer Council website.
First published in June 2016. This edition published in June 2021.
ISBN: 978 1 76096 154 1
Cancer Council Victoria and Department of Health Victoria 2021, Optimal care pathway for people with Acute Myeloid Leukaemia, 2nd edn, Cancer Council Victoria, Melbourne.
Enquiries about this publication can be sent to optimalcare.pathways@cancervic.org.au
Our thanks to the following health professionals, consumer representatives, stakeholders and organisations consulted in developing this optimal care pathway.
Professor Andrew Roberts (Chair), Haematologist and Bone Marrow Transplant Physician, The Royal Melbourne Hospital, Peter MacCallum Cancer Centre, The University of Melbourne and Walter and Eliza Hall Institute
Dr Chris Arthur, Haematologist, Royal North Shore Hospital
Dr Ashish Bajel, Haematologist and Bone Marrow Transplant Physician, The Royal Melbourne Hospital and Peter MacCallum Cancer Centre
Associate Professor Peter Bardy, Clinical Haematologist; Central Adelaide Local Health Network and Clinical Lead Bone Marrow Transplant; Royal Adelaide Hospital
Associate Professor Sidney Davis, Radiation Oncologist, Alfred Health
Professor John Gibson, Haematologist, Royal Prince Alfred Hospital Sydney and The University of Sydney
Ms Jennifer Hempton, Transplant and Apheresis Coordinator, Barwon Health
Dr David Kipp, Haematologist, University Hospital Geelong (Barwon Health) and Deakin University
Professor Robert Thomas, Special Advisor on Health, Professorial Fellow, The University of Melbourne
Professor Karin Thursky, Infectious Diseases Physician and Clinical Research Fellow, Doherty Institute, The Royal Melbourne Hospital and Peter MacCallum Cancer Centre
Associate Professor Andrew Wei, Haematologist, Alfred Health and Monash University
Ms Julia Brancato, Project Coordinator, Optimal Care Pathways, Cancer Council Victoria
Professor Andrew Roberts (Chair), Physician, Bone Marrow Transplantation Service, Department of Clinical Haematology and Medical Oncology, Clinical Haematologist, The Royal Melbourne Hospital
Associate Professor Andrew Wei, Haematologist, Alfred Health, AML Researcher, Monash University, Victorian Cancer Agency Research Fellow
Dr Ashish Bajel, Clinical Haematologist and Bone Marrow Transplant Physician, The Royal Melbourne Hospital, Peter MacCallum Cancer Centre
Dr Chris Arthur, Haematologist, Royal North Shore Hospital
Dr David Kipp, Clinical Haematologist, Andrew Love Cancer Centre, University Hospital Geelong
Darryl King, consumer representative
Dr Emma Cohen, Nurse Manager, Haematology, Oncology and Bone Marrow Transplant ward, Austin Health
Ms Helena Rodi, Clinical Dietitian, Austin Health
Professor John Gibson, Head of Haematology, Royal Prince Alfred Hospital, University of Sydney
Associate Professor Justin Tse, General Practitioner, Director of Medical Student’s Education, St Vincent’s Hospital Melbourne, The University of Melbourne
Ms Kaye Hose, Palliative Care Nurse Consultant, Austin Health
Ms Leanne Fagg, consumer representative
Dr Paul Cannell, Haematologist, Medical Co-Director, Fiona Stanley Hospital
Associate Professor Peter Bardy, Associate Clinical Professor, Haematology and Medical Oncology, Clinical Director of Cancer Services, Royal Adelaide Hospital
Professor Robert Thomas, Chief Advisor on Cancer, Department of Health and Human Services, Victoria
Dr Simon He, Clinical Haematologist and Bone Marrow Transplant Physician, Austin Health, The Royal Melbourne Hospital
Alexandra Viner, Project Manager – Optimal Care Pathways
Advance Care Planning Australia
Allied Health Professions Australia
Australasian Association of Nuclear Medicine Specialists
Australasian Chapter of Palliative Medicine, Royal Australia College of Physicians
Australasian Leukaemia and Lymphoma Group
Australian and New Zealand Society of Neuroradiology
Australian and New Zealand Society of Palliative Care
Australian Cancer Survivorship Centre
Australian College of Nursing
Australian Medical Association
Australian Society of Medical Imaging and Radiation Therapy
Bone Marrow Transplant Society of Australia and New Zealand
Cancer Nurses Society of Australia
Clinical Oncology Society of Australia
Haematology Society of Australia and New Zealand
Interventional Radiology Society of Australasia
Leukaemia Foundation
Medical Oncology Group of Australia
Oncology Social Workers Australia and New Zealand
Royal Australasian College of Physicians
Royal Australasian College of Surgeons
Royal Australian and New Zealand College of Radiologists
Royal Australian College of General Practitioners
Royal College of Pathologists of Australasia
Alfred Health
Cancer Australia
Cancer Council Victoria, Strategy and Support Division
Cancer Institute New South Wales
Concord Repatriation General Hospital New South Wales
Consumer representative
Department of Health Victoria, Commissioning and System Improvement Division, Cancer Unit
National Cancer Expert Reference Group
Olivia Newton-John Cancer Wellness and Research Centre
St Vincent’s Hospital Melbourne
Other stakeholders consulted to provide feedback include relevant Cancer Council committees and networks, Integrated Cancer Services, Primary Health Networks and several health services.
The multidisciplinary team will include the following members:
care coordinator (as determined by multidisciplinary team members)*
clinical haematologist *
infectious diseases physician*
nurse (with appropriate expertise or under the supervision of nurses with appropriate expertise)*
pathologist including pathologist with molecular genetic expertise*
pharmacist.*
The multidisciplinary team will often include some of the following members as appropriate for individual patient mix:
Aboriginal health practitioner, Indigenous liaison officer or remote general practitioner
clinical trials coordinator
dentist
dietitian
exercise physiologist
fertility specialist
general practitioner
genetic counsellor
geriatrician
nuclear medicine physician
occupational therapist
palliative care specialist
physiotherapist
psychiatrist
psychologist
radiation oncologist
radiologist/imaging specialists
social worker
spiritual/pastoral care.
* Denotes core members. Core members of the multidisciplinary team are expected to attend most multidisciplinary team meetings either in person or remotely.
American Cancer Society (ACS) 2018, ‘Risk factors for acute myeloid leukaemia (AML)’, viewed 28 January 2020 https://www.cancer.org/cancer/acute-myeloid-leukemia/causes-risks-prevention/risk-factors.html.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. 2016, ‘The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia’, Blood, vol. 127, no. 20, pp. 2391–2405.
Australian Adult Cancer Pain Management Guideline Working Party 2019, Australian adult cancer pain management guideline: ‘Cancer pain management in adults’, Cancer Council Australia, Sydney, viewed 6 June 2019, https://wiki.cancer.org.au/australiawiki/index.php?oldid=191646.
Australian Cancer Survivorship Centre 2016, ‘Survivorship care planning’, viewed 2 October 2020, https://www.petermac.org/sites/default/files/page/downloads/Survivorship_Care_Planning.pdf.
Australian Cancer Survivorship Centre 2019, Community support organisations’ cancer survivorship care consensus statement, viewed 10 February 2020, https://www.petermac.org/sites/default/files/media-uploads/NGO_ConsensusStatement.pdf.
Australian Clinical Trials 2015, ‘Potential benefits and risks’, National Health and Medical Research Council, Department of Industry, Innovation and Science, Australian Government, Canberra, viewed 24 July 2019, https://www.australianclinicaltrials.gov.au/why-be-part-clinical-trial/potential-benefits-and-potential-risks.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2014, Health literacy: taking action to improve safety and quality, ACSQHC, Sydney, viewed 18 February 2020, https://www.safetyandquality.gov.au/sites/default/files/migrated/Health-Literacy-Taking-action-to-improve-safety-and-quality.pdf.
Australian Commission for Safety and Quality in Health Care (ACSQHC) 2015, Credentialing health practitioners and defining their scope of clinical practice: a guide for managers and practitioners, ACSQHC, Sydney, viewed 18 February 2020, https://www.safetyandquality.gov.au/publications-and-resources/resource-library/credentialing-health-practitioners-and-defining-their-scope-clinical-practice-guide-managers-and-practitioners.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2017, National Safety and Quality Health Service Standards guide for hospitals, ACSQHC, Sydney, viewed 18 February 2020, https://www.safetyandquality.gov.au/wp-content/uploads/2017/12/National-Safety-and-Quality-Health-Service-Standards-Guide-for-Hospitals.pdf.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2019a, Person-centred care, ACSQHC, Sydney, viewed 15 May 2020, https://www.safetyandquality.gov.au/our-work/partnering-consumers/person-centred-care.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2019b, Australian Hospital Patient Experience Question Set, ACSQHC, Sydney, viewed 25 March 2020, https://www.safetyandquality.gov.au/our-work/indicators-measurement-and-reporting/australian-hospital-patient-experience-question-set.
Australian Commission for Safety and Quality in Health Care (ACSQHC) 2020, ‘NSQHS Standards user guide for medication management in cancer care’, ACSQHC, Sydney, viewed 16 April 2020, https://www.safetyandquality.gov.au/publications-and-resources/resource-library/nsqhs-standards-user-guide-medication-management-cancer-care.
Australian Health Ministers’ Advisory Council (AHMAC) 2011, A national framework for advance care directives, Council of Australian Governments Health Council, Adelaide, viewed 22 July 2019, http://www.coaghealthcouncil.gov.au/Portals/0/A%20National%20Framework%20for%20Advance%20Care%20Directives_September%202011.pdf.
Australian Institute of Health and Welfare (AIHW) 2016, Australia’s health 2016, Australia’s health series no. 15. Cat. no. AUS 199, AIHW, Canberra.
Australian Institute of Health and Welfare (AIHW) 2017, Access to health services by Australians with disability, viewed 20 February 2020, https://www.aihw.gov.au/reports/disability/access-health-services-disability/contents/content.
Australian Institute of Health and Welfare (AIHW) 2018, Australia’s health 2018, Australia’s health series no. 16. Cat. no. AUS 221, AIHW, Canberra.
Australian Institute of Health and Welfare (AIHW) 2019, Cancer in Australia 2019, Cancer series no. 119. Cat. No. CAN 123, AIHW, Canberra.
AYA Cancer Fertility Preservation Guidance Working Group 2014, Fertility preservation for AYAs diagnosed with cancer: guidance for health professionals, Cancer Council Australia, Sydney, viewed 20 July 2020, https://wiki.cancer.org.au/australia/COSA:AYA_cancer_fertility_preservation/Introduction.
Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, et al. 2005, ‘Prognostic index for adult patients with acute myeloid leukemia in first relapse’, Journal of Clinical Oncology, vol. 23, no. 9, pp. 1967–1978.
Buchbinder D, Kelly DL, Duarte RF, Auletta JJ, Bhatt N, Byrne M, et al. 2018, ‘Neurocognitive dysfunction in hematopoietic cell transplant recipients: expert review from the Late Effects and Quality of Life Working Committee of the CIBMTR and Complications and Quality of Life Working Party of the EBMT’, Bone Marrow Transplant, vol. 53, no. 5, pp. 535–555.
Cancer Australia 2015, National Aboriginal and Torres Strait Islander cancer framework, Cancer Australia, Surry Hills, viewed 24 August 2017, https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/national-aboriginal-and-torres-strait-islander-cancer-framework.
Cancer Australia 2017, Principles of cancer survivorship, Australian Government, Sydney, viewed 18 February 2020, https://canceraustralia.gov.au/sites/default/files/publications/principles-cancer-survivorship/pdf/pocs_-_principles_of_cancer_survivorship.pdf.
Cancer Australia 2019a, Principles of multidisciplinary care, Australian Government, Sydney, viewed 18 July 2019, https://canceraustralia.gov.au/system/tdf/guidelines/all_about_multidisciplinary_care.pdf?file=1&type=node&id=3551.
Cancer Australia 2019b, ‘Cancer incidence’, viewed 18 February 2020, https://ncci.canceraustralia.gov.au/diagnosis/cancer-incidence/cancer-incidence.
Cancer Australia 2020, Cancer care in the time of COVID-19: a conceptual framework for the management of cancer during a pandemic, Australian Government, Sydney, viewed 3 September 2020, https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/cancer-care-time-covid-19-conceptual-framework-management-cancer-during-pandemic.
Cancer Council Australia 2018, ‘Prevention’, Cancer Council Australia, Sydney, viewed 18 July 2019, https://www.cancer.org.au/preventing-cancer/.
Cancer Council Australia 2019, ‘Complementary and alternative therapies’, Cancer Council Australia, Sydney, viewed 22 July 2019, https://www.cancer.org.au/about-cancer/treatment/complementary-therapies-and-cancer.html.
Cancer Council Victoria 2019, ‘Palliative care’, Cancer Council Victoria, viewed 7 October 2019, https://www.cancervic.org.au/cancer-information/treatments/treatments-types/palliative_care/palliative-care-treatment.html.
Cancer Research in Primary Care 2016, Principles statement: Shared care, PC4 Shared Care Working Group, Melbourne, viewed 4 October 2019, http://pc4tg.com.au/wp-content/uploads/2016/07/PC4-Principles-Statement-shared-care-2016-1.pdf.
Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, et al. 1998, ‘Chemotherapy compared with autologous or allogeneic transplantation in the management of acute myeloid leukaemia in first remission’, New England Journal of Medicine, no. 339, pp. 1649–1656.
Clinical Oncology Society of Australia (COSA) 2013, Special Issue: COSA’s 40th Annual Scientific Meeting, Cancer Care Coming of Age, 12–14 November 2013, Adelaide Convention Centre, Asia-Pacific Journal of Clinical Oncology, vol. 9, no. 3, pp. 61–98.
Clinical Oncology Society of Australia (COSA) 2014, Psychosocial management of AYAs diagnosed with cancer: guidance for health professionals, COSA, Sydney, viewed 7 October 2019, http://wiki.cancer.org.au/australia/COSA:Psychosocial_management_of_AYA_cancer_patients.
Clinical Oncology Society of Australia (COSA) 2015, Cancer care coordinator: position statement, COSA, Sydney, viewed 22 July 2019, https://www.cosa.org.au/media/332296/cancer-care-coordinator-position-statement_final-endorsed-by-council_161115_cnsa-logo.pdf.
Clinical Oncology Society of Australia (COSA) 2016, Australasian tele-trial model: access to clinical trials closer to home using tele-health a national guide for implementation, v1.7, COSA, Sydney, viewed 18 July 2019, https://www.cosa.org.au/media/332325/cosa-teletrial-model-final-19sep16.pdf.
Clinical Oncology Society of Australia (COSA) 2018, COSA position statement on exercise in cancer care, COSA, Sydney, viewed 22 July 2019, https://www.cosa.org.au/media/332488/cosa-position-statement-v4-web-final.pdf.
Commonwealth Department of Health 2017, Out-of-pocket expenses for private medical treatment (informed financial consent), Commonwealth of Australia, Canberra.
Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, et al. 2018, ‘Clinical Oncology Society of Australia position statement on exercise in cancer care’, Medical Journal of Australia, vol. 209, no. 4, pp. 184–187.
Cormie P, Zopf EM, Zhang X, Schmitz KH 2017, ‘The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects’, Epidemiologic Reviews, vol. 39, no. 1, pp. 71–92.
Davis JR, Benjamin DJ, Jonas BA 2018, ‘New and emerging therapies for acute myeloid leukaemia’, Journal of Investigative Medicine, vol. 66, pp. 1088–1095.
Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. 2015, ‘Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations’, Annals of Oncology, vol. 26, no.1, pp. 288–300.
Department of Human Services (DHS) 2020, ‘Leukaemia – acute myeloid leukaemia’, viewed 29 January 2020, https://www.humanservices.gov.au/organisations/health-professionals/services/medicare/written-authority-required-drugs/drug-program-or-condition/leukaemia-acute-myeloid-leukaemia.
DiNardo CD, Wei AH 2020, ‘How I treat acute myeloid leukemia in the era of new drugs’, Ash Publications, vol. 135, no. 2, pp. 85–96.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, et al. 2010, ‘Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet’, Blood, vol. 115, no. 3, pp. 453–474.
Döhner H, Estey E, Grimwalde D, Amadori S, Appelbaum FR, et al. 2017, ‘Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel’, Blood, vol. 129, no. 4, pp. 424–447.
Döhner H, Weisdorf DJ, Bloomfield CD 2015, ‘Acute myeloid leukemia’, The New England Journal of Medicine, vol. 373, no. 12, pp. 1136–1152.
eviQ 2019a, ‘Safe handling and waste management of hazardous drugs’, Cancer Institute NSW, Sydney, viewed 22 July 2019, https://www.eviq.org.au/clinical-resources/administration-of-antineoplastic-drugs/188-safe-handling-and-waste-management-of-hazardou.
eviQ 2019b, ‘Acute myeloid leukaemia induction/consolidation/maintenance with midostaurin treatment overview’, viewed 29 January 2020, https://www.eviq.org.au/haematology-and-bmt/leukaemias/acute-myeloid-leukaemia/3520-acute-myeloid-leukaemia-induction-consolidati.
Emery J 2014, ‘Cancer survivorship – the role of the GP’, Australian Family Physician, vol. 43, no. 8, pp. 521–525.
Ferrari A, Thomas D, Franklin A, Hayes-Lattin B, Mascarin M, van der Graaf W, et al. 2010, ‘Starting an adolescent and young adult program: some success stories and some obstacles to overcome’, Journal of Clinical Oncology, vol. 28, no. 32, pp. 4850–4857.
Fitch M 2000, ‘Supportive care for cancer patients’, Hospital Quarterly, vol. 3, no. 4, pp. 39–46.
Fitch MI 2008, ‘Supportive care framework’, Canadian Oncology Nursing Journal, vol. 18, no. 1, pp. 6–14.
Ganzel C, Mathews V, Alimoghaddam K, Ghavamzadeh A, Kuk D, Devlin S, et al. 2016, ‘Autologous transplant remains the preferred therapy for relapsed APL in CR2’, Bone Marrow Transplantation, vol. 51, no. 9, pp. 1180–1183.
Gilligan T, Coyle N, Frankel RM, Berry DL, Bohlke K, Epstein, RM, et al. 2017, ‘Patient–clinician communication: American Society of Clinical Oncology consensus guideline’, Journal of Clinical Oncology, vol. 35, no. 31, pp. 3618–3623.
Griggs J, Mangu P, Anderson H, Balaban E, Dignam J, Hryniuk W, et al. 2012, ‘Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline’, Journal of Clinical Oncology, vol. 30, no. 13, pp. 1553–1561.
Grimwade D, Ivey A, Juntly BJP 2016, ‘Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance’, Medical Journal of Australia, vol. 194, no. 3, pp. 107–108.
Gorin NC, Labopin M, Frassoni F, Milpied N, Attal M, Blaise D, et al. 2008, ‘Identical outcome after autologous or allogeneic genoidentical hematopoietic stem-cell transplantation in first remission of acute myelocytic leukemia carrying inversion 16 or t(8; 21): a retrospective study from the European Cooperative Group for Blood and Marrow Transplantation’, Journal of Clinical Oncology, vol. 26, no. 19, pp. 3183–3188.
Hack TF, Reuther DJ, Weir LM, Grenier D, Degner LF 2012, ‘Promoting consultation recording practice in oncology: identification of critical implementation factors and determination of patient benefit’, Psycho-Oncology, vol. 22, pp. 1273–1282.
Haines IE 2011, ‘Managing patients with advanced cancer: the benefits of early referral for palliative care’, Medical Journal of Australia, vol. 194, no. 3, pp. 107–108.
Hayes SC, Newton RU, Spence RR, Galvao DA 2019, ‘The Exercise and Sports Science Australia position statement: exercise medicine in cancer management’, Journal of Science and Medicine in Sport, vol. 22, no. 11, pp. 1175–1199.
Hewitt M, Greenfield S, Stovall E 2006, From cancer patient to cancer survivor: lost in transition, National Academies Press, Washington.
Hilgendorf I, Greinix H, Halter JP, Lawitschka A, Bertz H, Wolff D 2015, ‘Long-term follow-up after allogeneic stem cell transplantation’, Deutsches Ärzteblatt International, vol. 112, pp. 51–58.
Holter Chakrabarty JL, Rubinger M, Le-Rademacher J, Wang HL, Grigg A, Selby GB, et al. 2014, ‘Autologous is superior to allogeneic hematopoietic cell transplantation for acute promyelocytic leukemia in second complete remission’, Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation, vol. 20, no. 7, pp. 1021–1025.
Jefford M, Ward AC, Lisy K, Lacey K, Emery JD, Glaser AW, et al. 2017, ‘Patient-reported outcomes in cancer survivors: a population-wide cross-sectional study’, Support Care Cancer, no. 10, pp. 3171–3179.
Jones WC, Parry C, Devine S, Main DS, Sasaki SO, Tran ZV 2015, ‘Prevalence and predictors of distress in post-treatment adult leukemia and lymphoma survivors’, Journal of Psychosocial Oncology, vol. 33, no. 2, pp. 124–141.
Laidsaar-Powell R, Butow P, Boyle F, Juraskova 2018a, ‘Facilitating collaborative and effective family involvement in the cancer setting: guidelines for clinicians (TRIO guidelines-1)’, Patient Education and Counseling, vol. 101, no. 6, pp. 970–982.
Laidsaar-Powell R, Butow P, Boyle F, Juraskova 2018b, ‘Managing challenging interactions with family caregivers in the cancer setting: guidelines for clinicians (TRIO guidelines-2)’, Patient Education and Counselling, vol. 101, no. 6, pp. 983–994.
Lengfelder E, Lo-Coco F, Ades L, Montesinos P, Grimwade D, Kishore B, et al. 2015, ‘Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European LeukemiaNet’, Leukemia, vol. 29, no. 5, pp. 1084–1091.
Lisy K, Langdon L, Piper A, Jefford M 2019, ‘Identifying the most prevalent unmet needs of cancer survivors in Australia: a systematic review’, Asia-Pacific Journal of Clinical Oncology, vol. 15, no. 5, pp. e68–e78.
McMahon CM, Perl AE 2019, ‘Management of primary refractory acute myeloid leukemia in the era of targeted therapies’, Leukemia and Lymphoma, vol. 60, no. 3, pp. 583–597.
Monterosso L, Platt V, Bulsara M, Berg M 2019, ‘Systematic review and meta-analysis of patient reported outcomes for nurse-led models of survivorship care for adult cancer patients’, Cancer Treatment Reviews Journal, no. 73, pp. 62–72.
My Health Record 2019, ‘What is My Health Record?’ Australian Government, Sydney, viewed 17 December 2019, https://www.myhealthrecord.gov.au/for-you-your-family/what-is-my-health-record.
National Cancer Institute (NCI) 2020, Adult acute myeloid leukaemia treatment (PDQ) – health professional version, viewed 29 January 2020, https://www.cancer.gov/types/leukemia/hp/adult-aml-treatment-pdq#_AboutThis_1.
National Cancer Survivorship Initiative (NCSI) 2015, ‘Stratified pathways of care’, National Health Service, UK, viewed 2 March 2015.
National Comprehensive Cancer Network (NCCN) 2015, NCCN clinical practice guidelines in oncology – acute myeloid leukemia, NCCN 20th annual edition, version I, 2015, NCCN, Plymouth Meeting, PA.
National Comprehensive Cancer Network (NCCN) 2019, NCCN clinical practical guidelines in oncology: acute myeloid leukaemia, version 3.2020, NCCN, Plymouth Meeting, PA.
National Disability Insurance Agency 2018, ‘How the NDIS works’, NDIS, Canberra, viewed 3 June 2019, https://www.ndis.gov.au/understanding/how-ndis-works.
National Health and Medical Research Council (NHMRC) 2013, Personalised medicine and genetics, viewed 3 June 2019, https://www.nhmrc.gov.au/about-us/publications/personalised-medicine-and-genetics.
National Health and Medical Research Council (NHMRC) 2014, Talking with your patients about complementary medicine: a resource for clinicians, NHMRC, Canberra, viewed 18 February 2020, https://www.caresearch.com.au/caresearch/ClinicalPractice/PatientConsiderations/ComplementaryTherapies/tabid/1258/Default.aspx.
palliAGED 2018, ‘Needs Assessment’, Flinders University, Bedford Park, viewed 1 October 2019, https://www.palliaged.com.au/tabid/4879/Default.aspx.
Palliative Care Australia 2018, National Palliative Care Standards, 5th edn, Palliative Care Australia, Canberra, viewed 24 July 2019, http://palliativecare.org.au/wp-content/uploads/dlm_uploads/2018/11/PalliativeCare-National-Standards-2018_Nov-web.pdf.
Palliative Care Victoria 2019, ‘Spiritual care’, Palliative Care Victoria, Melbourne, viewed 22 July 2019, https://www.pallcarevic.asn.au/healthcare-professionals/about-palliative-care/care/spiritual-care/.
Perl A, Martinelli G, Cortes J, Neubauer A, Berman E, Paolini S, et al. 2019, ‘Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML’, New England Journal of Medicine, vol. 381. no. 18, pp. 1728–1740.
Peter MacCallum Cancer Centre 2019, Community support organisations’ cancer survivorship care consensus statement, viewed 10 February 2020, https://www.petermac.org/sites/default/files/media-uploads/NGO_ConsensusStatement.pdf.
Queensland Health 2015, Sad news sorry business: guidelines for caring for Aboriginal and Torres Strait Islander people through death and dying, State Government of Queensland, Brisbane, viewed 22 July 2019, https://www.health.qld.gov.au/__data/assets/pdf_file/0023/151736/sorry_business.pdf.
Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, et al. 2018, ‘Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party’, Blood, vol. 131, no. 12, pp. 1275–1291.
Sharafeldin N, Bosworth A, Patel SK, Chen Y, Morse E, Mather M, et al. 2018, ‘Cognitive functioning after hematopoietic cell transplantation for hematologic malignancy: results from a prospective longitudinal study’, Journal of Clinical Oncology, vol. 36, no. 5, pp. 463–475.
Silver JK, Baima J 2013, ‘Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes’, American Journal of Physical Medicine & Rehabilitation, vol. 92, no. 8, pp. 715–727.
Silver JK 2015, ‘Cancer prehabilitation and its role in improving health outcomes and reducing health care costs’, Seminars in Oncology Nursing, vol. 31, no. 1, pp. 1–3.
Sjoquist K, Zalcberg J 2013, ‘Clinical trials – advancing cancer care’, Cancer Forum, vol. 37, no. 1, pp. 80–88.
Smith A, Bellizzi K, Keegan T, Zebrack B, Chen V, Neale A, et al. 2013, ‘Health-related quality of life of adolescent and young adult patients with cancer in the United States: the Adolescent and Young Adult Health Outcomes and Patient Experience Study’, Journal of Clinical Oncology, vol. 31, no. 17, pp. 2136–2145.
Smith S, Case L, Waterhouse K, Pettitt N, Beddard L, Oldham J, et al. 2012, A blueprint of care for teenagers and young adults with cancer, Teenage Cancer Trust, Manchester, UK.
Sorror ML, Storer BE, Fathi AT, Gerds AT, Medeiros BC 2017, ‘Development and validation of a novel acute myeloid leukemia-composite model to estimate risks of mortality’, The Journal of the American Medical Association, vol. 3, no. 12, pp. 1675–1682.
Steer B, Marx G, Singhal N, McJannett M, Goldstein D, Prowse R 2009, ‘Cancer in older people: a tale of two disciplines’, Internal Medicine Journal, vol. 39, pp. 771–775.
Tan SY, Turner J, Kerin-Ayres K, Butler S, Deguchi C, Khatri S, et al. 2019, ‘Health concerns of cancer survivors after primary anti-cancer treatment’, Support Cancer Care, no. 10, pp. 3739–3747.
Temel J, Greer J, Muzikansky A, Gallagher E, Admane S, Jackson V 2010, ‘Early palliative care for patients with non-metastatic non-small cell lung cancer’, New England Journal of Medicine, vol. 363, no. 8, pp. 733–742.
Vardy JL, Raymond JC, Koczwara B, Lisy K, Cohn RJ, Joske D, et al. 2019, ‘Clinical Oncology Society of Australia position statement on cancer survivorship care’, Australian Journal of General Practice, vol. 48, no.12, pp. 833–836.
Venditti A, Piciocchi A, Candoni A, Melillo L, Calafiore V, Cairoli R, et al. 2019, ‘GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia’, Blood, vol. 134, no. 12, pp. 935–945.
Vijayvergia N, Denlinger CS 2015, ‘Lifestyle factors in cancer survivorship: where we are and where we are headed’, Journal of Personalized Medicine, vol. 5, no. 3, pp. 243–263.
Wildiers H, Heeren P, Puts M, Topinkova E, Maryska LG, Janssen-Heijnen MLG, et al. 2014, ‘International Society of Geriatric Oncology Consensus on geriatric assessment in older patients with cancer’, Journal of Clinical Oncology, vol. 32, no. 24, pp. 2595–2603.
Wingelhofer B, Somervaille TCP 2019, ‘Emerging epigenetic therapeutic targets in acute myeloid leukemia’, Frontiers in Oncology, vol. 9, pp. 850.
World Health Organization (WHO) 2018, ‘Malnutrition’, WHO, Geneva, viewed 18 July 2019, https://www.who.int/news-room/fact-sheets/detail/malnutrition.
Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. 2014, ‘Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial’, Lancet, vol. 383, no. 9930, pp. 1721–1730.