Optimal timeframes & summary
Timeframes to treatment: Timeframes should be informed by evidence-based guidelines (where they exist) while recognising that shorter timelines for appropriate consultations and treatment can reduce people’s distress. The following recommended timeframes are based on expert advice from the Cancer of Unknown Primary Working Group.
| Step in pathway | Care point | Timeframe |
| Presentation, initial investigations and referral | 2.3 Referral | Patients should be seen by a specialist within two weeks of a referral from their general practitioner. |
| Diagnosis, staging and treatment planning | 3.1 Diagnostic work-up | Investigations should be completed within two weeks. |
| Treatment | 4.2 Treatment options | Treatment should start within two weeks of the
decision to treat. |
The optimal care pathway outlines seven critical steps in the patient journey. While the seven steps appear in a linear model, in practice, patient care does not always occur in this way but depends on the particular situation (such as the type of cancer, when and how the cancer is diagnosed, prognosis, management, patient decisions and the patient’s physiological response to treatment).
The pathway describes the optimal cancer care that should be provided at each step.
Special considerations
CUP is a distinct clinical entity. In Australia in 2014, CUP was the13th most commonly diagnosed cancer and in 2016 the sixth leading cause of cancer death. The number of new cases of CUP diagnosed in Australia increased from 2,141 in 1982 to 2,666 in 2014. Over the same time period, the age-standardised incidence rate decreased from 18 cases per 100,000 people in 1982 to 9.7 cases per 100,000 people in 2014 (AIHW 2018).
The term ‘cancer of unknown primary’ refers to a metastatic malignancy for which a standardised diagnostic work-up fails to identify the site of origin. CUP is a very heterogeneous disease in which the type of tumour, the extent of disease and the outcome of treatment all vary widely (NICE 2010). The entry point for this optimal care pathway is when patients present with a metastatic malignancy without an obvious primary site. Many of these patients will have a malignancy of epithelial lineage. The term CUP in this care pathway refers to patients with tumours of epithelial, neuroendocrine or undifferentiated lineage with no known primary site.
Malignancies of definitive non-epithelial lineage, such as melanoma, sarcoma and lymphoma, form a minority group of CUP where management can be undertaken without identifying a primary site; therefore, these malignancies are not specifically included in this pathway. Once a patient is diagnosed with melanoma, sarcoma or lymphoma, they should be treated according to existing tumour-specific pathways.
Diagnosing CUP is a continuum from initial presentation with the results of limited tests, to a final diagnosis after all relevant investigations have been completed. CUP may be suspected when a patient presents with metastatic malignancy on clinical examination or by imaging without an obvious primary site. CUP is confirmed when metastatic epithelial, neuroendocrine or undifferentiated malignancy is identified on the basis of final histology, where a reasonable amount of investigations have failed to identify a primary site with certainty (NICE 2010).
Some patients are given a label of ‘suspected CUP’ based on their clinical picture and radiological findings without any histopathological examination of the tumour having occurred because they may be assessed as being too unwell at presentation to undergo any form of further investigation or treatment. Hence the actual diagnosis of these patients is in fact unknown. These patients are still considered throughout this pathway and fit into the subset of patients with unfavourable prognosis as described below.
CUP subsets
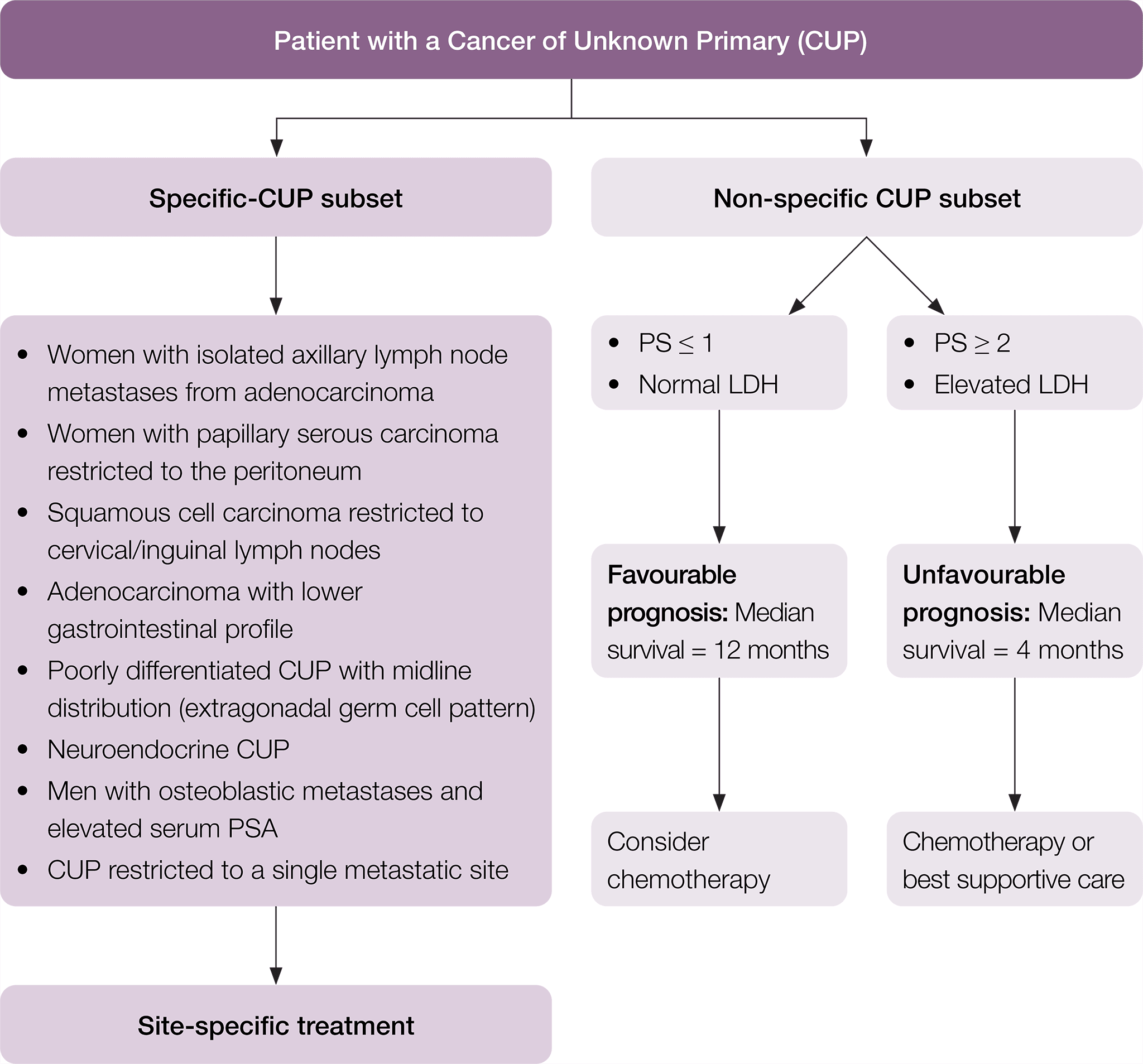
Among patients diagnosed with CUP, specific subsets can be identified based on histopathological and clinical manifestations (Pavlidis et al. 2015). In particular, there is a specific clinico-pathological subset of patients with favourable prognosis that have a pattern of disease similar to known cancer types and may respond well to standard disease-specific treatment. The remaining patients are considered within the non-specific subset of CUP, with their prognosis and suitability for treatment dependent on their Eastern Cooperative Oncology Group (ECOG) performance status and lactate dehydrogenase (LDH) level. Where appropriate to do so, this pathway has been divided into these subgroups of patients. In this pathway, the following definitions are used.
Specific clinico-pathological subsets of CUP include (Fizazi et al. 2015, Pavlidis & Fizazi 2005, Pavlidis & Pentheroudakis 2012, Vajdic & Goldstein 2015):
- poorly differentiated neuroendocrine carcinoma of unknown primary
- well-differentiated neuroendocrine tumour of unknown primary
- peritoneal adenocarcinomatosis of a serous papillary histological type in females
- isolated axillary nodal metastases in females
- squamous cell carcinoma involving non-supraclavicular cervical lymph nodes
- isolated inguinal adenopathy (squamous carcinoma)
- CUP with an intestinal phenotype and immunohistochemistry (IHC) (including CK20+/CDX2+/CK7−) or molecular profile
- single metastatic deposit from unknown primary
- men with blastic bone metastases or IHC/serum prostate specific antigen (PSA) expression
- poorly differentiated carcinoma with midline distribution (extragonadal germ cell syndrome).
Non-specific clinico-pathological subsets of CUP include patients with CUP who do not belong to one of the specific CUP subsets. Examples include (Pavlidis et al. 2015, Vajdic & Goldstein 2015):
- adenocarcinoma metastatic to the liver or other organs
- poorly differentiated carcinoma
- non-papillary malignant ascites (adenocarcinoma)
- multiple cerebral metastases (adeno or squamous carcinoma)
- multiple lung/pleural metastases (adenocarcinoma)
- multiple bone metastases (adenocarcinoma)
- squamous abdominopelvic CUP
- undifferentiated malignancy that cannot be further classified.
Non-specific CUP patients may be considered to be of (Fizazi et al. 2015):
- favourable prognosis if they have a good ECOG performance score (0–1) and a normal serum LDH (the median life expectancy is around one year)
- Unfavourable prognosis if they have either a poor ECOG performance score (≥ 2) and/or elevated serum LDH (the median life expectancy is around four months).
* Fizazi K, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2015; 26 (suppl_5): v133–v138 doi:10.1093/annonc/mdv305. Adapted and reproduced with permission of Oxford University Press on behalf of ESMO. Oxford University Press and ESMO are not responsible or in any way liable for the accuracy of the adaptation, for any errors, omissions or inaccuracies, or for any consequences arising therefore. Cancer Institute NSW is solely responsible for the adapted material in this work. Please visit the ESMO Cancers of Unknown Primary Site website