4.2 Treatment options
The advantages and disadvantages of each treatment and associated potential side effects should be discussed with the patient and their carer/family.
Treatment should be individualised according to the clinico-pathological subset and the suspected primary site. The following treatment recommendations have been adapted from the ESMO guidelines (Fizazi et al. 2015).
A suggested flow chart to guide treatment is provided.
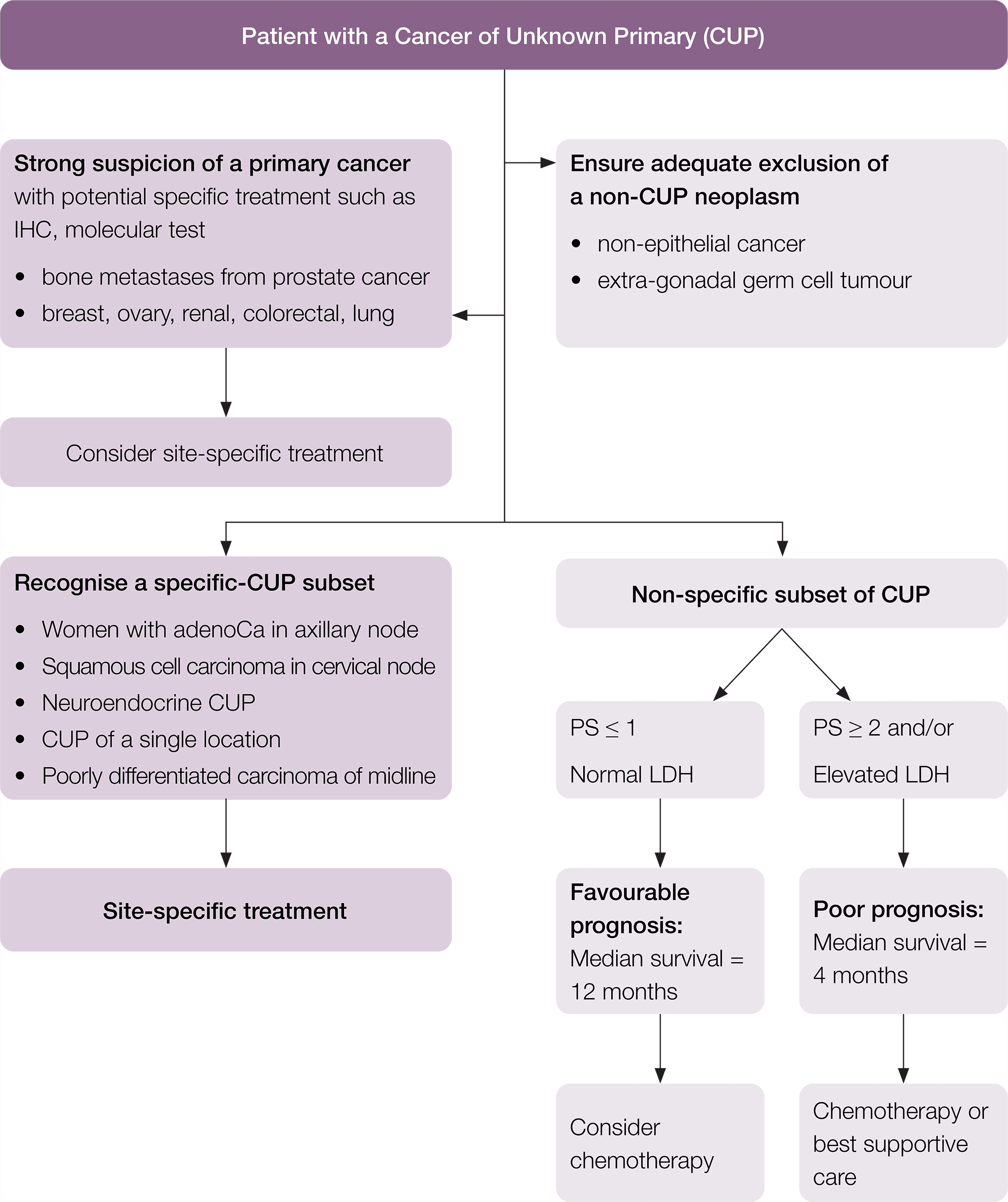
* Fizazi K, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2015; 26 (suppl_5): v133–v138 doi:10.1093/annonc/mdv305. Adapted and reproduced with permission of Oxford University Press on behalf of ESMO. Oxford University Press and ESMO are not responsible or in any way liable for the accuracy of the adaptation, for any errors, omissions or inaccuracies, or for any consequences arising therefore. Cancer Institute NSW is solely responsible for the adapted material in this work. Please visit the ESMO Cancers of Unknown Primary Site website.
Patients in the specific-CUP subset who have a good prognosis should be treated the same as patients with equivalent known primary tumours with metastatic disease, as shown in Table 1.
|
Equivalent known primary tumour |
Recommended treatment |
|
Poorly differentiated neuroendocrine carcinoma of unknown primary |
Treat as poorly differentiated neuroendocrine carcinomas with a known primary |
|
Well-differentiated neuroendocrine tumour of unknown primary |
Treat as well-differentiated neuroendocrine tumour of a known primary site |
|
Peritoneal adenocarcinomatosis of a serous papillary histological type in females |
Treat as ovarian cancer |
|
Isolated axillary nodal adenocarcinoma metastases in females |
Treat as breast cancer |
|
Squamous cell carcinoma involving non-supraclavicular cervical lymph nodes |
Treat as head and neck squamous cell cancer |
|
CUP with an intestinal phenotype and IHC (CK20+/CDX2+/CK7−) or molecular profile |
Treat as metastatic colorectal cancer |
|
Single metastatic deposit from unknown primary |
Treat as single metastases by resection or high-dose (ablative) radiotherapy depending on the location |
|
Osteoblastic bone metastases or IHC/serum PSA expression in men |
Treat as prostate cancer |
|
Patients with extragonadal germ cell syndrome |
Treat as poor-prognosis germ cell tumour (Greco 2013). |
|
Isolated inguinal adenopathy (squamous carcinoma) |
Local dissection with or without local radiotherapy (Pavlidis et al. 2015) |
Other tumour specific optimal care pathways can be found here.
* Fizazi K, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology 2015; 26 (suppl_5): v133–v138 doi:10.1093/annonc/mdv305. Adapted and reproduced with permission of Oxford University Press on behalf of ESMO. Oxford University Press and ESMO are not responsible or in any way liable for the accuracy of the adaptation, for any errors, omissions or inaccuracies, or for any consequences arising therefore. Cancer Institute NSW is solely responsible for the adapted material in this work. Please visit the ESMO Cancers of Unknown Primary Site website.
For patients with a non-specific subset of CUP, but who have a favourable prognosis, a two-drug chemotherapy regimen as per the NCCN or ESMO guidelines should be considered (Culine et al. 2003, Gross-Goupil et al. 2012, Hainsworth et al. 2010).
Patients with localised disease may be suitable for local therapies such as high-dose (ablative) radiotherapy (Janssen et al. 2014) or surgical excision.
CUP patients identified in the poor-prognosis non-specific group can be considered for treatment with low-toxicity, palliative, chemotherapy regimens and/or best supportive care (Fizazi et al. 2015).
Using palliative radiotherapy to relieve local symptoms should also be considered where appropriate (Rich & Mendenhall 2016, Tey et al. 2017). In addition, other palliative procedures to assist in symptom control may also be considered in specific situations such as video-assisted thoracoscopic surgery pleurodesis or PleurX (if available) for interventional pain relief.
Timeframe for commencing treatment
Timeframes for diagnosis should be informed by evidence-based guidelines (where they exist) while recognising that shorter timelines for appropriate consultations and treatment can reduce patient distress.
Treatment of CUP should begin within two weeks of the decision to treat (four weeks from referral).







