Colorectal cancer
Optimal care pathways map seven key steps in cancer care. Each of these steps outlines nationally agreed best practice for the best level of care. While the seven steps appear in a linear model, in practice, patient care does not always occur in this way but depends on the particular situation (e.g. the type of cancer, when and how the cancer is diagnosed, prognosis, management, the patient’s decisions and their physiological response to treatment).
The principles underpinning optimal care pathways always put patients at the centre of care throughout their experience and prompt the healthcare system to deliver coordinated care.
The optimal care pathways do not constitute medical advice or replace clinical judgement, and they refer to clinical guidelines and other resources where appropriate.

Evidence-based guidelines, where they exist, should inform timeframes. Treatment teams need to recognise that shorter timeframes for appropriate consultations and treatment can promote a better experience for patients. Three steps in the pathway specify timeframes for care. They are designed to help patients understand the timeframes in which they can expect to be assessed and treated, and to help health services plan care delivery in accordance with expert-informed time parameters to meet the expectation of patients. These timeframes are based on expert advice from the Colorectal Cancer Working Group.
Timeframes for care
| Step in pathway | Care point | Timeframe |
| Screening participation – National Bowel Cancer Screening Program (NBCSP) at-home bowel test is recommended for average risk and asymptomatic people aged 50–74 years, every 2 years. People should be encouraged to enter their GP’s details on the NBCSP form so the GP also receives their test results. | ||
| Presentation, initial investigations and referral | Signs and symptoms | Presenting symptoms should be promptly and clinically triaged with a health professional |
| Initial investigations initiated by GP | Test results should be provided to the patient within 1 week of testing. | |
| Referral to specialist | If symptoms suggest colorectal cancer, patients should be referred and colonoscopy completed within 4 weeks
The patient should see a surgeon within 2 weeks of GP referral following a positive diagnosis of colorectal cancer via colonoscopy |
|
| Diagnosis, staging and treatment planning | Diagnosis and staging | Investigations should be completed within 2 weeks. 10–15% of colorectal cancers will present as an emergency; this necessitates appropriate acute care followed by multidisciplinary management |
| Multidisciplinary meeting and treatment planning | MDM should take place within 2 weeks of diagnosis and staging | |
| Treatment | Surgery | Colorectal cancer – surgery should be completed within 5 weeks of investigations and MDM if no neoadjuvant therapy required
Rectal cancer with neoadjuvant therapy – surgery should be completed 8–12 weeks after completion of neoadjuvant therapy |
| Neoadjuvant radiation therapy | Neoadjuvant radiation therapy should begin within 3 weeks of the MDM | |
| Systemic therapy | Neoadjuvant chemotherapy should begin within 3 weeks of the MDM
Adjuvant chemotherapy should begin within 8 weeks of surgery |
|
Seven steps of the optimal care pathway
Step 1: Prevention and early detection
Step 2: Presentation, initial investigations and referral
Step 3: Diagnosis, staging and treatment planning
Step 4: Treatment
Step 5: Care after initial treatment and recovery
Step 6: Managing recurrent, residual or metastatic disease
Step 7: End-of-life care
Colorectal cancer was the third most commonly diagnosed cancer in Australia in 2016. It is estimated that it will be the fourth most commonly diagnosed cancer in 2020 (Cancer Australia 2020b). Bowel cancer is the second highest cause of death after lung cancer. In 2019, it was estimated that the risk of an individual being diagnosed with colorectal cancer by their 85th birthday would be one in 14 (one in 12 males and one in 17 females) (Cancer Australia 2019b).
This step outlines recommendations for the prevention and early detection of colorectal cancer.
Evidence shows that not smoking, avoiding or limiting alcohol intake, eating a healthy diet, maintaining a healthy body weight, being physically active, being sun smart and avoiding exposure to oncoviruses or carcinogens may help reduce cancer risk (Cancer Council Australia 2018).
Recommendations for reducing the risk of colorectal cancer include:
- completing the National Bowel Cancer Screening Program (NBCSP) at-home bowel cancer test every two years if aged 50–74 years
- eating a healthy diet, including plenty of vegetables, fruit and whole grains while minimising intake of red meat, barbequed/grilled meat and processed meat
- maintaining a healthy body weight
- undertaking regular physical activity
- avoiding or limiting alcohol intake
- not smoking.
For all people aged 50–70 years, including those at average risk of colorectal cancer, aspirin should be actively considered to prevent colorectal cancer, in conjunction with other comorbidities. A low dose (100–300 mg per day) is recommended for at least 2.5 years (Cancer Council Australia Colorectal Cancer Guidelines Working Party 2019). Benefit for cancer prevention (though shorter for cardiovascular risk) is evident only 10 years after initiation, so a life expectancy of at least 10 years should be taken into consideration in the advice to use aspirin (Cancer Council Australia Colorectal Cancer Guidelines Working Party 2019).
Based on family history, people can be placed into one of three categories (described below) of relative risk of developing colorectal cancer. Age is an independent risk factor for colorectal cancer. These categories were developed by the Cancer Council Australia Colorectal Cancer Guidelines Working Party (2019).
General practitioners and primary care nurses should educate patients and encourage them to participate in the screening appropriate to the patient’s level of risk.
Category 1: Near average risk
Risk factors for category 1 patients include:
- no first- or second-degree relative with colorectal cancer
- one first-degree relative with colorectal cancer diagnosed before the age of 55
- one first-degree and one second-degree relative with colorectal cancer diagnosed at 55 years or older.
People who have one relative with colorectal cancer diagnosed at age 55 or older should be advised that their own risk of developing colorectal cancer could be up to twice the average risk but is still not high enough to justify colorectal cancer screening by colonoscopy.
Category 2: Moderately increased risk
People in this category should be advised that their risk of developing colorectal cancer is at least three times higher than average but could be up to six times higher than average, if they have any of the following:
- one first-degree relative with colorectal cancer diagnosed before the age of 55
- two first-degree relatives with colorectal cancer at any age
- one first-degree relative and at least two second-degree relatives diagnosed with colorectal cancer at any age.
Category 3: High risk
People in this category should be advised that their risk of colorectal cancer is at least seven times higher than average but could be up to 10 times higher than average, if any of the following apply:
- at least three first-degree relatives diagnosed with colorectal cancer at any age
- at least three first-degree or second-degree relatives with colorectal cancer, with at least one diagnosed before age 55 years
- they are a member of a family in which a gene mutation that confers a high risk of bowel cancer has been identified.
The NBCSP invites people starting at age 50 and continuing to age 74 (at average risk and asymptomatic) to screen for bowel cancer using a free, simple test at home.
NBCSP participants with screen-detected bowel cancer are more likely to be diagnosed at a less advanced stage than those who did not participate (Cancer Council Australia 2020). NBCSP participants with screen-detected bowel cancer had a lower risk of bowel cancer mortality compared with those who were diagnosed outside of the program (9.6 per cent vs 23.8 per cent) (AIHW 2018b). Bowel cancer screening on a population basis significantly reduces mortality and morbidity (Cancer Council Australia 2020).
Cancer Council Australia Colorectal Cancer Guidelines Working Party (2019) recommends the following.
Category 1: Near average risk
For people with category 1 risk of colorectal cancer, an immunochemical faecal occult blood test (iFOBT) should be performed every two years from age 50 to age 74.
Full examination of the large bowel by colonoscopy is recommended for people who have a positive iFOBT.
Category 2: Moderately increased risk
For people with category 2 risk of colorectal cancer, offer iFOBT every two years starting at age 40, then colonoscopy every five years starting at age 50. CT colonography may be offered if colonoscopy is contraindicated.
Category 3: High risk
For people with category 3 risk of colorectal cancer, offer iFOBT every two years starting at age 35, then colonoscopy every five years from age 45 to age 74. CT colonography may be offered if colonoscopy is not feasible.
Consider referral to a familial cancer service for further risk assessment and possible genetic testing.
Refer to a bowel cancer specialist to plan appropriate surveillance and management.
First-degree relatives of people with colorectal cancer who fall into the category 2 (moderate risk) group (where a relative is diagnosed under 55 years of age or where there are multiple first-degree relatives with colorectal cancer) should be advised to see their general practitioner with a view to referral for a colonoscopy if appropriate.
All other relatives should be advised to follow the average risk guidelines, including participating in the National Bowel Cancer Screening Program.
The following signs, symptoms and results should be investigated:
- positive iFOBT
- passage of blood with or without mucus in the faeces
- unexplained iron deficiency anaemia
- change in bowel habit (loose stools or constipation), especially a recent one that does not have another explanation such as an infection or opioids
- undiagnosed abdominal pain or tenderness
- unexplained rectal or abdominal mass
- unexplained weight loss (Cancer Council Australia Colorectal Guidelines Working Party 2019)
- lethargy.
The presence of multiple signs and symptoms, particularly in combination with other underlying risk factors, indicates an increased risk of colorectal cancer.
Presenting symptoms should be promptly and clinically triaged with a health professional.
General practitioner examinations and investigations should include:
- physical examination
- digital rectal examination
- full blood examination and iron studies (Cancer Council Australia Colorectal Guidelines Working Party 2019).
Note: a negative result from an iFOBT does not exclude cancer.
A detailed family history should be obtained from patients presenting with possible symptoms of colorectal cancer.
Test results should be provided to the patient within one week of testing.
All patients referred for colonoscopy should be seen by a specialist accredited in colonoscopy by the Conjoint Committee of the Royal Australasian College of Surgeons, Royal Australasian College of Physicians and Gastroenterological Society of Australia) to make the diagnosis.
Patients should be enabled to make informed decisions about their choice of specialist and health service. General practitioners should make referrals in consultation with the patient after considering the clinical care needed, cost implications (see referral options and informed financial consent), waiting periods, location and facilities, including discussing the patient’s preference for health care through the public or the private system.
Referral for suspected or diagnosed colorectal cancer should include the following essential information to accurately triage and categorise the level of clinical urgency:
- important psychosocial history and relevant medical history
- family history, current symptoms, medications and allergies
- results of current clinical investigations (imaging and pathology reports)
- results of all prior relevant investigations
- notification if an interpreter service is required.
Many services will reject incomplete referrals, so it is important that referrals comply with all relevant health service criteria.
If access is via online referral, a lack of a hard copy should not delay referral.
The specialist should provide timely communication to the general practitioner about the consultation and should notify the general practitioner if the patient does not attend appointments.
If a pathological (or endoscopic) diagnosis has been made, the patient should be referred to a general or colorectal surgeon affiliated with (or with access to) a multidisciplinary team. Some early cancers can be managed by endoscopy alone without surgical consultation but should also be considered by a multidisciplinary team.
Aboriginal and Torres Strait Islander patients will need a culturally appropriate referral. To view the optimal care pathway for Aboriginal and Torres Strait Islander people and the corresponding quick reference guide, visit the Cancer Australia website. Download the consumer resources – Checking for cancer and Cancer from the Cancer Australia website.
If symptoms suggest colorectal cancer, patients should be referred and a colonoscopy completed within four weeks of the general practitioner referral.
Patients should be seen by the surgeon within two weeks of the general practitioner referral following a positive diagnosis of colorectal cancer via colonoscopy. Patients should bring a copy of the colonoscopy report and other relevant medical and psychosocial history.
The patient’s general practitioner should consider an individualised supportive care assessment where appropriate to identify the needs of an individual, their carer and family. Refer to appropriate support services as required. See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific needs may arise for patients at this time:
- assistance for dealing with the emotional distress and/or anger of dealing with a potential cancer diagnosis, anxiety/depression, interpersonal problems and adjustment difficulties
- management of physical symptoms including pain, fatigue and altered bowel function
- encouragement and support to increase levels of exercise (Cormie et al. 2018; Hayes et al. 2019).
For more information refer to the NICE 2015 guidelines, Suspected cancer: recognition and referral.
For additional information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
The general practitioner is responsible for:
- providing patients with information that clearly describes to whom they are being referred, the reason for referral and the expected timeframes for appointments
- requesting that patients notify them if the specialist has not been in contact within the expected timeframe
- considering referral options for patients living rurally or remotely
- supporting the patient while waiting for the specialist appointment (Cancer Council nurses are available to act as a point of information and reassurance during the anxious period of awaiting further diagnostic information; patients can contact 13 11 20 nationally to speak to a cancer nurse).
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
Step 3 outlines the process for confirming the diagnosis and stage of cancer and for planning subsequent treatment. The guiding principle is that interaction between appropriate multidisciplinary team members should determine the treatment plan.
The treatment team, after taking a thorough medical history and making a thorough medical examination of the patient, should undertake the following investigations under the guidance of a specialist.
Patients thought to have resectable or potentially curable disease should have routine blood tests (full blood count [FBC], urea and electrolytes [U&E], liver function tests [LFT], carcinoembryonic antigen [CEA]), a complete colonic assessment by colonoscopy, or virtual colonoscopy in the case of an impassable tumour plus the following, as clinically indicated:
For colon and rectal cancer:
- computed tomography (CT) scan of the chest, abdomen and pelvis
- if CT shows metastatic disease confined to the liver, MRI of the liver to assess for resectability
- PET-CT – this is recommended for patients who have suspicious metastatic disease at initial staging. It also recommended for restaging for those who have potentially resectable/treatable metastatic disease.
For rectal cancer:
- endorectal ultrasound to assess T1 and early T2 tumours in patients who may be appropriate for local resection techniques.
It is essential that the treating surgeon accurately assesses the tumour location.
Investigations should be completed within two weeks.
Between 10 and 15 per cent of colorectal cancers will present as an emergency. This necessitates appropriate acute care followed by management from a multidisciplinary team.
Between 1 and 5 per cent of colorectal cancers are specifically inherited (familial adenomatous polyposis and lynch syndrome) and up to 25 per cent may have some inherited component. The features that suggest a genetic predisposition may include:
- early age at onset
- multiple primary cancers
- multiple polyps
- the histological type of polyp(s)
- family history of similar or related cancers.
Universal testing for mismatch repair deficiency by IHC or MSI testing is now recommended as standard practice for treatment-related and familial risk assessments.
Anyone diagnosed with cancer should have a detailed personal and family cancer history taken. People with a strong family history of colorectal cancer or high-risk familial syndromes should be referred to a familial cancer service for genetic risk assessment and possible genetic screening of affected relatives. Younger patients under 50 years should be referred to a familial cancer service. Consult relevant guidelines to determine if referral to a familial cancer service is appropriate.
Mainstream genetic testing is now available and funded through Medicare for the mismatch repair genes, APC, MUTYH and the hamartomatous polyposis genes. However, a familial cancer service assessment can assist and determine if genetic testing is appropriate. Genetic testing should be offered when there is at least a 10 per cent chance of finding a causative ‘gene error’ (pathogenic gene variant; previously called a mutation). Tumour testing for mismatch repair (MMR) protein expression or microsatellite testing is the main avenue to determining this risk for lynch syndrome. Usually testing begins with a variant search in a person who has had cancer (a diagnostic genetic test). If a pathogenic gene variant is identified, variant-specific testing is available to relatives to see if they have inherited the familial gene variant (predictive genetic testing). Depending on the personal and family history, the relevant state health system may support funding in the public sector for additional germline testing.
Pre-test counselling and informed consent is required before any genetic testing in any setting. A familial cancer service can organise this, as well as provide risk management advice, facilitate family risk notification and arrange predictive cascade genetic testing for the family.
Visit the Centre for Genetics Education website for basic information about cancer in a family.
For detailed information and referral guidelines for colorectal cancer risk assessment and consideration of genetic testing, see these resources:
Pharmacogenetics describes how individual genetic differences can lead to differences in the way certain medicines interact with the body. These interactions can affect the effectiveness of medications and any side effects. Applying pharmacogenetics to treatment planning may help patients to be prescribed the most appropriate treatment at the optimal dose from the beginning of treatment (NHMRC 2013).
Staging is a critical element in treatment planning and should be clearly documented in the patient’s medical record.
Preoperative staging, including the investigations listed in section 3.1, is used to define the extent of tumour spread and to determine neoadjuvant therapy use.
- Clinical pathological staging will occur after surgery and is needed to inform post-treatment care.
- Synoptic reporting is recommended.
- Screen for loss of expression of MMR protein in all patients at the time of biopsy and after tumour resection.
Visit the Cancer Institute New South Wales website for information about understanding the stages of cancer.
Patient performance status is a central factor in cancer care and should be clearly documented in the patient’s medical record.
Performance status should be measured and recorded using an established scale such as the Karnofsky scale or the Eastern Cooperative Oncology Group (ECOG) scale.
A number of factors should be considered at this stage:
- the patient’s overall condition, life expectancy, personal preferences and decision- making capacity
- discussing the multidisciplinary team approach to care with the patient
- appropriate and timely referral to an MDM
- pregnancy and fertility
- support with travel and accommodation
- teleconferencing or videoconferencing as required.
The multidisciplinary team should meet to discuss newly diagnosed patients within two weeks of diagnosis and staging so that a treatment plan can be recommended and there can be early preparation for the post-treatment phase. The level of discussion may vary, depending on the patient’s clinical and supportive care factors. Some patients with non-complex cancers may not be discussed by a multidisciplinary team; instead the team may have treatment plan protocols that will be applied if the patient’s case (cancer) meets the criteria. If patients are not discussed at an MDM, they should at least be named on the agenda for noting. The proposed treatment must be recorded in the patient’s medical record and should be recorded in an MDM database where one exists.
Teams may agree on standard treatment protocols for non-complex care, facilitating patient review (by exception) and associated data capture.
Results of all relevant tests and access to images should be available for the MDM. Information about the patient’s concerns, preferences and social and cultural circumstances should also be available.
The multidisciplinary team requires administrative support in developing the agenda for the meeting, for collating patient information and to ensure appropriate expertise around the table to create an effective treatment plan for the patient. The MDM has a chair and multiple lead clinicians. Each patient case will be presented by a lead clinician (usually someone who has seen the patient before the MDM). In public hospital settings, the registrar or clinical fellow may take this role. A member of the team records the outcomes of the discussion and treatment plan in the patient history and ensures these details are communicated to the patient’s general practitioner. The team should consider the patient’s values, beliefs and cultural needs as appropriate to ensure the treatment plan is in line with these.
The multidisciplinary team should be composed of the core disciplines that are integral to providing good care. Team membership should reflect both clinical and supportive care aspects of care. Pathology and radiology expertise are essential.
See ‘About this OCP’ for a list of team members who may be included in the multidisciplinary team for colorectal cancer.
Core members of the multidisciplinary team are expected to attend most MDMs either in person or remotely via virtual mechanisms. Additional expertise or specialist services may be required for some patients. An Aboriginal and Torres Strait Islander cultural expert should be considered for all patients who identify as Aboriginal or Torres Strait Islander.
The general practitioner who made the referral is responsible for the patient until care is passed to another practitioner who is directly involved in planning the patient’s care.
The general practitioner may play a number of roles in all stages of the cancer pathway including diagnosis, referral, treatment, shared follow-up care, post-treatment surveillance, coordination and continuity of care, as well as managing existing health issues and providing information and support to the patient, their family and carer.
A nominated contact person from the multidisciplinary team may be assigned responsibility for coordinating care in this phase. Care coordinators are responsible for ensuring there is continuity throughout the care process and coordination of all necessary care for a particular phase (COSA 2015). The care coordinator may change over the course of the pathway.
The lead clinician is responsible for overseeing the activity of the team and for implementing treatment within the multidisciplinary setting.
Patients should be encouraged to participate in research or clinical trials where available and appropriate.
For more information visit the Cancer Australia website.
Cancer prehabilitation uses a multidisciplinary approach combining exercise, nutrition and psychological strategies to prepare patients for the challenges of cancer treatment such as surgery, systemic therapy and radiation therapy. Team members may include anaesthetists, oncologists, surgeons, haematologists, clinical psychologists, exercise physiologists, physiotherapists and dietitians, among others.
Patient performance status is a central factor in cancer care and should be frequently assessed. All patients should be screened for malnutrition using a validated tool, such as the Malnutrition Screening Tool (MST). The lead clinician may refer obese or malnourished patients to a dietitian preoperatively or before other treatments begin.
Patients who currently smoke should be encouraged to stop smoking before receiving treatment. This should include an offer of referral to Quitline in addition to smoking cessation pharmacotherapy if clinically appropriate.
Evidence indicates that patients who respond well to prehabilitation may have fewer complications after treatment. For example, those who were exercising before diagnosis and patients who use prehabilitation before starting treatment may improve their physical or psychological outcomes, or both, and this helps patients to function at a higher level throughout their cancer treatment (Cormie et al. 2017; Silver 2015).
For patients with colorectal cancer, the multidisciplinary team should consider these specific prehabilitation assessments and interventions for treatment-related complications or major side effects:
- conducting a physical and psychological assessment to establish a baseline function level
- identifying impairments and providing targeted interventions to improve the patient’s function level (Silver & Baima 2013)
- referral to a stoma nurse
- reviewing the patient’s medication to ensure optimisation and to improve adherence to medicine used for comorbid conditions.
Following completion of primary cancer treatment, rehabilitation programs have considerable potential to enhance physical function.
Cancer and cancer treatment may cause fertility problems. This will depend on the age of the patient, the type of cancer and the treatment received. Infertility can range from difficulty having a child to the inability to have a child. Infertility after treatment may be temporary, lasting months to years, or permanent (AYA Cancer Fertility Preservation Guidance Working Group 2014).
Patients need to be advised about and potentially referred for discussion about fertility preservation before starting treatment and need advice about contraception before, during and after treatment. Patients and their family should be aware of the ongoing costs involved in optimising fertility. Fertility management may apply in both men and women. Fertility preservation options are different for men and women and the need for ongoing contraception applies to both men and women.
A diagnosis of colorectal cancer in reproductive-age women may carry a risk of future infertility. Therefore, the risk of infertility, and fertility preservation options, both before and after cancer therapy, should be discussed with patients and their families as part of the initial cancer management planning. This discussion should be ongoing, with firm follow-up arrangements put into place. All discussions should be documented in the patient’s medical record.
Treatment for colorectal cancer may reduce female fertility through damage to the ovaries (from chemotherapy or radiation), damage to the uterus (from radiation) and potentially through damage to the fallopian tubes from abdominal surgery. Chemotherapy, especially with alkylating agents, is likely to reduce ovarian reserve, leading to a reduced fertility window or premature menopause. The type of chemotherapy will determine the extent of infertility associated with follicle loss. Radiation of the pelvis commonly induces permanent ovarian failure and may damage the uterus such that future pregnancy confers risk to the mother and fetus.
Pelvic radiation may also induce vaginal adhesions or stenosis, limiting future sexual activity. This risk can be reduced by using vaginal cylinders and topical oestrogen treatment.
Fertility preservation interventions including egg or embryo freezing and ovarian tissue cryopreservation offer realistic options for having a family, although sometimes egg donation and surrogacy may ultimately be required. Women of reproductive age who are concerned about future fertility should be referred to a fertility specialist to discuss potential fertility preservation interventions. Women with early menopause due to colorectal cancer treatment should be offered hormone replacement therapy and (if possible) managed by a menopause specialist.
Some colorectal cancers may be associated with a genetic risk, and specific gene testing in IVF embryos may reduce transmission risk. Some gastrointestinal cancers may be associated with a high risk of other cancers including uterine cancers. Careful counselling and surveillance are required, especially if future pregnancy is a consideration.
An early, collaborative and multidisciplinary approach with the Medical Services Advisory Committee and the surgical, oncology and fertility preservation teams will maximise the opportunity for best practice care and consideration for future fertility for young women diagnosed with colorectal cancer.
See the Cancer Council website for more information.
See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific challenges and needs may arise for patients at this time:
- assistance for dealing with psychological and emotional distress while adjusting to the diagnosis; treatment phobias; existential concerns; stress; difficulties making treatment decisions; anxiety or depression or both; psychosexual issues such as potential loss of fertility and premature menopause; history of sexual abuse; and interpersonal problems
- management of physical symptoms such as pain, fatigue (Australian Adult Cancer Pain Management Guideline Working Party 2019), weight loss and altered bowel function
- malnutrition or undernutrition, identified using a validated nutrition screening tool such as the MST (note that many patients with a high BMI [obese patients] may also be malnourished [WHO 2018])
- pre-surgical education with a stomal therapy nurse wherever a temporary or permanent stoma is a possibility
- guidance for financial and employment issues (e.g. loss of income, travel and accommodation)
- requirements for rural patients and caring arrangements for other family members
- support for families or carers who are distressed with the patient’s cancer diagnosis
- support for families/relatives who may be distressed after learning of a genetically linked cancer diagnosis
- specific spiritual needs that may benefit from the involvement of pastoral/spiritual care
- low self-esteem and disturbed body image, which are more prevalent in stoma patients than in non-stoma patients.
Between 32 and 44 per cent of patients report psychological distress following a diagnosis of colorectal cancer, and patients with stomas are at higher risk (El-Shami et al. 2015; Cancer Council Australia Colorectal Cancer Guidelines Working Party 2019). This large number indicates a need for screening patients to identify those at high risk of anxiety or depression at each visit. Provide patients with tailored and accurate information before treatment, and facilitate patient decision making about appearance-altering treatment and meeting others with a similar personal experience (Hong et al. 2014). Consider pre-surgical referral to a psycho-oncologist for support over body image expectations associated with surgical treatment.
Additionally, palliative care may be required at this stage.
For more information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
In discussion with the patient, the lead clinician should undertake the following:
- establish if the patient has a regular or preferred general practitioner and, if the patient does not have one, then encourage them to find one
- provide written information appropriate to the health literacy of the patient about the diagnosis and treatment to the patient and carer and refer the patient to the Guide to best cancer care (consumer optimal care pathway) for bowel cancer, as well as to relevant websites and support groups as appropriate
- provide a treatment care plan including contact details for the treating team and information on when to call the hospital
- discuss a timeframe for diagnosis and treatment with the patient and carer
- discuss the benefits of multidisciplinary care and gain the patient’s consent before presenting their case at an MDM
- provide brief advice and refer to Quitline (13 7848) for behavioural intervention if the patient currently smokes (or has recently quit), and prescribe smoking cessation pharmacotherapy, if clinically appropriate
- recommend an ‘integrated approach’ throughout treatment regarding nutrition, exercise and minimal or no alcohol consumption among other considerations
- communicate the benefits of continued engagement with primary care during treatment for managing comorbid disease, health promotion, care coordination and holistic care
- where appropriate, review fertility needs with the patient and refer for specialist fertility management (including fertility preservation, contraception, management during pregnancy and of future pregnancies)
- be open to and encourage discussion about the diagnosis, prognosis (if the patient wishes to know) and survivorship and palliative care while clarifying the patient’s preferences and needs, personal and cultural beliefs and expectations, and their ability to comprehend the communication
- encourage the patient to participate in advance care planning including considering appointing one or more substitute decision-makers and completing an advance care directive to clearly document their treatment preferences. Each state and territory has different terminology and legislation surrounding advance care directives and substitute decision-makers.
The lead clinician has these communication responsibilities:
- involving the general practitioner from the point of diagnosis
- ensuring regular and timely communication with the general practitioner about the diagnosis, treatment plan and recommendations from MDMs and inviting them to participate in MDMs (consider using virtual mechanisms)
- supporting the role of general practice both during and after treatment
- discussing shared or team care arrangements with general practitioners or regional cancer specialists, or both, together with the patient.
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
Step 4 describes the optimal treatments for colorectal cancer, the training and experience required of the treating clinicians and the health service characteristics required for optimal cancer care.
All health services must have clinical governance systems that meet the following integral requirements:
- identifying safety and quality measures
- monitoring and reporting on performance and outcomes
- identifying areas for improvement in safety and quality (ACSQHC 2020).
Step 4 outlines the treatment options for colorectal cancer. For detailed clinical information on treatment options refer to these resources:
- the Clinical practice guidelines for the prevention, early detection and management of colorectal cancer
- European Society of Medical Oncology Clinical Practice Guidelines: Gastrointestinal cancers
- National Comprehensive Cancer Network ‘Colorectal Cancer Screening’
- The American Society for Radiation Oncology ‘Appropriate Customization of Radiation Therapy for Stage II and III Rectal Cancer: an ASTRO Clinical Practice Statement Using the RAND/UCLA Appropriateness Method
The intent of treatment can be defined as one of the following:
- curative
- anti-cancer therapy to improve quality of life or longevity (or both) without expectation of cure
- symptom palliation.
The treatment intent should be established in a multidisciplinary setting, documented in the patient’s medical record and conveyed to the patient and carer as appropriate.
The potential benefits need to be balanced against the morbidity and risks of treatment.
The lead clinician should discuss the advantages and disadvantages of each treatment and associated potential side effects with the patient and their carer or family before treatment consent is obtained and begins so the patient can make an informed decision. Supportive care services should also be considered during this decision-making process. Patients should be asked about their use of (current or intended) complementary therapies (see Appendix D).
Timeframes for starting treatment should be informed by evidence-based guidelines where they exist. The treatment team should recognise that shorter timeframes for appropriate consultations and treatment can promote a better experience for patients.
Initiate advance care planning discussions with patients before treatment begins (this could include appointing a substitute decision-maker and completing an advance care directive). Formally involving a palliative care team/service may benefit any patient, so it is important to know and respect each person’s preference (AHMAC 2011).
Surgery is recommended for many patients diagnosed with colorectal cancer.
Timeframe for starting treatment
- Colorectal cancer – surgery should be completed within five weeks of completing investigations and the MDM if no neoadjuvant therapy is required.
- Rectal cancer with neoadjuvant therapy – surgery should be completed in eight to 12 weeks after completing neoadjuvant therapy.
Training and experience required of the surgeon
- Surgeon (FRACS or equivalent) with adequate training and experience and institutional cross-credentialing and the agreed scope of practice within this area.
- Specifically, for rectal surgery, surgeons must be sub-specialists with an appropriate level of training and experience and treat an appropriate caseload annually.
Documented evidence of the surgeon’s training and experience, including their specific (sub-specialty) experience with colorectal cancer and procedures to be undertaken, should be available.
Health service characteristics
To provide safe and quality care for patients having surgery, health services should have these features:
- stomal therapy support
- critical care support
- 24-hour medical staff availability
- 24-hour operating room access and intensive care unit
- diagnostic imaging
- pathology
- nuclear medicine imaging.
Some patients may benefit from radiation therapy:
- patients with high-risk rectal cancer (neoadjuvant therapy)
- patients with symptomatic, non-resectable locally advanced rectal cancer who may benefit from radiation therapy with or without concurrent chemotherapy given with palliative intent
- patients with colon cancer where the tumour has penetrated a fixed structure.
Timeframe for starting treatment
Neoadjuvant radiation therapy should begin within three weeks of the MDM.
Training and experience required of the appropriate specialists
The appropriate specialist should be a radiation oncologist (FRANZCR) with adequate training and experience with the agreed scope of practice in colorectal cancer.
The training and experience of the radiation oncologist should be documented.
Health service unit characteristics
To provide safe and quality care for patients having radiation therapy, health services should have these features:
- linear accelerator (LINAC) capable of image-guided radiation therapy (IGRT)
- dedicated CT planning
- access to MRI and PET imaging
- automatic record-verify of all radiation treatments delivered
- a treatment planning system
- trained medical physicists, radiation therapists and nurses with radiation therapy experience
- coordination for combined therapy with systemic therapy, especially where facilities are not co-located
- participation in Australian Clinical Dosimetry Service audits
- an incident management system linked with a quality management system.
Some patients may benefit from systemic therapy:
- those at high risk of relapse and who may benefit from adjuvant therapy
- those with locally advanced (high-risk) rectal cancer, treated with neoadjuvant chemoradiation therapy
- those with non-resectable, locally advanced or metastatic disease.
Timeframes for starting treatment
- Neoadjuvant chemotherapy should begin within three weeks of the MDM.
- Adjuvant chemotherapy should begin within eight weeks of surgery.
Training and experience required of the appropriate specialists
Medical oncologists must have training and experience of this standard:
- Fellow of the Royal Australian College of Physicians (or equivalent)
- adequate training and experience that enables institutional credentialing and agreed scope of practice within this area (ACSQHC 2015).
Cancer nurses should have accredited training in these areas:
- anti-cancer treatment administration
- specialised nursing care for patients undergoing cancer treatments, including side effects and symptom management
- the handling and disposal of cytotoxic waste (ACSQHC 2020).
Systemic therapy should be prepared by a pharmacist whose background includes this experience:
- adequate training in systemic therapy medication, including dosing calculations according to protocols, formulations and/or preparation.
In a setting where no medical oncologist is locally available (e.g. regional or remote areas), some components of less complex therapies may be delivered by a general practitioner or nurse with training and experience that enables credentialing and agreed scope of practice within this area. This should be in accordance with a detailed treatment plan or agreed protocol, and with communication as agreed with the medical oncologist or as clinically required.
The training and experience of the appropriate specialist should be documented.
Health service characteristics
To provide safe and quality care for patients having systemic therapy, health services should have these features:
- a clearly defined path to emergency care and advice after hours
- access to diagnostic pathology including basic haematology and biochemistry, and imaging
- cytotoxic drugs prepared in a pharmacy with appropriate facilities
- occupational health and safety guidelines regarding handling of cytotoxic drugs, including preparation, waste procedures and spill kits (eviQ 2019)
- guidelines and protocols to deliver treatment safely (including dealing with extravasation of drugs)
- coordination for combined therapy with radiation therapy, especially where facilities are not co-located
- appropriate molecular pathology access.
4.2.4 Targeted therapies and immunotherapy
There are several targeted therapies that may be used as part of treatment for colorectal cancer. These include bevacizumab and cetuximab, among others. They are only used for metastatic disease in conjunction with chemotherapy.
There is a role for immunotherapy in selected metastatic cancer cases and currently only in microsatellite unstable tumours.
4.2.5 Emerging therapies
The key principle for precision medicine is prompt and clinically oriented communication and coordination with an accredited laboratory and pathologist. Tissue analysis is integral for access to emerging therapies and, as such, tissue specimens should be treated carefully to enable additional histopathological or molecular diagnostic tests in certain scenarios.
There is an increasing role for molecular pathology including RAS status, BRAF status and MSI status, which will influence treatment options.
MMR testing should be done routinely on all primary tumours. Methylation testing for tumours showing MLH1 loss should be undertaken if available. Methylation effectively excludes lynch syndrome.
For metastatic disease, molecular pathology is important including RAS status, BRAF status and MMR.
4.3 Palliative care
Early referral to palliative care can improve the quality of life for people with cancer and in some cases may be associated with survival benefits (Haines 2011; Temel et al. 2010; Zimmermann et al. 2014). This is particularly true for cancers with poor prognosis.
The lead clinician should ensure patients receive timely and appropriate referral to palliative care services. Referral should be based on need rather than prognosis. Emphasise the value of palliative care in improving symptom management and quality of life to patients and their carers.
The ‘Dying to Talk’ resource may help health professionals when initiating discussions with patients about future care needs (see ‘More information’). Ensure that carers and families receive information, support and guidance about their role in palliative care (Palliative Care Australia 2018).
Patients, with support from their family or carer and treating team, should be encouraged to consider appointing a substitute decision-maker and to complete an advance care directive.
Refer to Step 6 for a more detailed description of managing patients with recurrent, residual or metastatic disease.
These online resources are useful:
The team should support the patient to participate in research or clinical trials where available and appropriate. Many emerging treatments are only available on clinical trials that may require referral to certain trial centres.
For more information visit the Cancer Australia website.
See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific challenges and needs may arise for patients at this time:
- assistance for dealing with emotional and psychological issues, including body image concerns, fatigue, quitting smoking, traumatic experiences, existential anxiety, treatment phobias, anxiety/depression, interpersonal problems and sexuality concerns
- potential isolation from normal support networks, particularly for rural patients who are staying away from home for treatment
- managing physical symptoms such as pain, weight loss, fatigue, altered bowel function and/or diarrhoea (patients can talk about managing these symptoms with specialist nurses such as a stomal therapy nurse or continence nurse)
- gastrointestinal symptoms (e.g. nausea, vomiting, mucositis and loss of appetite) as a result of chemotherapy, requiring optimal symptom control with medication and referral to a dietitian if dietary intake is affected
- odours and flatus arising from stomas, faecal or urinary fistulae (referral to continence nurses or stomal therapy nurses may be useful)
- assistance with managing complex medication regimens, multiple medications, assessment of side effects and assistance with difficulties swallowing medications – referral to a pharmacist may be required
- decline in mobility or functional status as a result of treatment
- reduced sexual interest and sexual dysfunction – particularly in stoma patients compared with patients with intact sphincters (Reese et al. 2014) – may require referral to medical specialists. Sensitive discussion and referral to a clinician skilled in this area may be appropriate
- erectile dysfunction and ejaculation dysfunction, which require sensitive discussion; referral to a clinician skilled in this area may be appropriate (Averyt & Nishimoto 2014)
- fertility issues for patients of reproductive age; refer to a fertility specialist before starting treatment
- assistance with beginning or resuming regular exercise with referral to an exercise physiologist or physiotherapist (COSA 2018; Hayes et al. 2019).
Early involvement of general practitioners may lead to improved cancer survivorship care following acute treatment. General practitioners can address many supportive care needs through good communication and clear guidance from the specialist team (Emery 2014).
Patients, carers and families may have these additional issues and needs:
- financial issues related to loss of income (through reduced capacity to work or loss of work) and additional expenses as a result of illness or treatment
- advance care planning, which may involve appointing a substitute decision-maker and completing an advance care directive
- legal issues (completing a will, care of dependent children) or making an insurance, superannuation or social security claim on the basis of terminal illness or permanent disability.
Cancer Council’s 13 11 20 information and support line can assist with information and referral to local support services.
For more information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
Rehabilitation may be required at any point of the care pathway. If it is required before treatment, it is referred to as prehabilitation (see section 3.6.1).
All members of the multidisciplinary team have an important role in promoting rehabilitation. Team members may include occupational therapists, speech pathologists, dietitians, social workers, psychologists, physiotherapists, exercise physiologists and rehabilitation specialists.
To maximise the safety and therapeutic effect of exercise for people with cancer, all team members should recommend that people with cancer work towards achieving, and then maintaining, recommended levels of exercise and physical activity as per relevant guidelines. Exercise should be prescribed and delivered under the direction of an accredited exercise physiologist or physiotherapist with experience in cancer care (Vardy et al. 2019). The focus of intervention from these health professionals is tailoring evidence-based exercise recommendations to the individual patient’s needs and abilities, with a focus on the patient transitioning to ongoing self-managed exercise.
Other issues that may need to be dealt with include managing cancer-related fatigue, improving physical endurance, achieving independence in daily tasks, optimising nutritional intake, returning to work and ongoing adjustment to cancer and its sequels. Referrals to dietitians, psychosocial support, return-to-work programs and community support organisations can help in managing these issues.
The lead or nominated clinician should take responsibility for these tasks:
- discussing treatment options with patients and carers, including the treatment intent and expected outcomes, and providing a written version of the plan and any referrals
- providing patient and carers with information about the possible side effects of treatment, managing symptoms between active treatments, how to access care, self-management strategies and emergency contacts
- encouraging patients to use question prompt lists and audio recordings, and to have a support person present to aid informed decision making
- initiating a discussion about advance care planning and involving carers or family if the patient wishes.
The general practitioner plays an important role in coordinating care for patients, including helping to manage side effects and other comorbidities, and offering support when patients have questions or worries. For most patients, simultaneous care provided by their general practitioner is very important.
The lead clinician, in discussion with the patient’s general practitioner, should consider these points:
- the general practitioner’s role in symptom management, supportive care and referral to local services
- the patient’s general practitioner may have expertise with many conditions that arise during treatment
- the patient’s general practitioner may have specific knowledge about the patient or access to local resources to help with prehabilitation, rehabilitation psychosocial needs and family issues
- using a chronic disease management plan and mental health care management plan
- how to ensure regular and timely two-way communication about:
- the treatment plan, including intent and potential side effects
- supportive and palliative care requirements
- the patient’s prognosis and their understanding of this
- enrolment in research or clinical trials
- changes in treatment or medications
- the presence of an advance care directive or appointment of a substitute decision-maker
- recommendations from the multidisciplinary team.
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
The term ‘cancer survivor’ describes a person living with cancer, from the point of diagnosis until the end of life. Survivorship care in Australia has traditionally been provided to patients who have completed active treatment and are in the post-treatment phase. But there is now a shift to provide survivorship care and services from the point of diagnosis to improve cancer-related outcomes.
Cancer survivors may experience inferior quality of life and cancer-related symptoms for up to five years after their diagnosis (Jefford et al. 2017). Distress, fear of cancer recurrence, fatigue, obesity and sedentary lifestyle are common symptoms reported by cancer survivors (Vardy et al. 2019).
Due to an ageing population and improvements in treatments and supportive care, the number of people surviving cancer is increasing. International research shows there is an important need to focus on helping cancer survivors cope with life beyond their acute treatment. Cancer survivors often face issues that are different from those experienced during active treatment for cancer and may include a range of issues, as well as unmet needs that affect their quality of life (Lisy et al. 2019; Tan et al. 2019).
Physical, emotional and psychological issues include fear of cancer recurrence, cancer-related fatigue, pain, distress, anxiety, depression, cognitive changes and sleep issues (Lisy et al. 2019). Late effects may occur months or years later and depend on the type of cancer treatment. Survivors and their carers may experience impacted relationships and practical issues including difficulties with return to work or study and financial hardship. They may also experience changes to sex and intimacy. Fertility, contraception and pregnancy care after treatment may require specialist input.
The Institute of Medicine, in its report From cancer patient to cancer survivor: Lost in transition, describes the essential components of survivorship care listed in the paragraph above, including interventions and surveillance mechanisms to manage the issues a cancer survivor may face (Hewitt et al. 2006). Access to a range of health professions may be required including physiotherapy, occupational therapy, social work, dietetics, clinical psychology, fertility and palliative care. Coordinating care between all providers is essential to ensure the patient’s needs are met.
Cancer survivors are more likely than the general population to have and/or develop comorbidities (Vijayvergia & Denlinger 2015). Health professionals should support survivors to self-manage their own health needs and to make informed decisions about lifestyle behaviours that promote wellness and improve their quality of life (Australian Cancer Survivorship Centre 2016; Cancer Australia 2017; NCSI 2015).
The transition from active treatment to post-treatment care is critical to long-term health. In some cases, people will need ongoing, hospital-based care, and in other cases a shared follow-up care arrangement with their general practitioner may be appropriate. This will vary depending on the type and stage of cancer and needs to be planned.
Shared follow-up care involves the joint participation of specialists and general practitioners in the planned delivery of follow-up and survivorship care. A shared care plan is developed that outlines the responsibilities of members of the care team, the follow-up schedule, triggers for review, plans for rapid access into each setting and agreement regarding format, frequency and triggers for communication.
After completing initial treatment, a designated member of the multidisciplinary team (most commonly nursing or medical staff involved in the patient’s care) should provide the patient with a needs assessment and treatment summary and develop a survivorship care plan in conjunction with the patient. This should include a comprehensive list of issues identified by all members of the multidisciplinary team involved in the patient’s care and by the patient. These documents are key resources for the patient and their healthcare providers and can be used to improve communication and care coordination.
The treatment summary should cover, but is not limited to:
- the diagnostic tests performed and results
- diagnosis including stage, prognostic or severity score
- tumour characteristics
- treatment received (types and dates)
- current toxicities (severity, management and expected outcomes)
- interventions and treatment plans from other health providers
- potential long-term and late effects of treatment
- supportive care services provided
- follow-up schedule
- contact information for key healthcare providers.
Responsibility for follow-up care should be agreed between the lead clinician, the general practitioner, relevant members of the multidisciplinary team and the patient. This is based on guideline recommendations for post-treatment care, as well as the patient’s current and anticipated physical and emotional needs and preferences.
Evidence comparing shared follow-up care and specialised care indicates equivalence in outcomes including recurrence rate, cancer survival and quality of life (Cancer Research in Primary Care 2016).
Ongoing communication between healthcare providers involved in care and a clear understanding of roles and responsibilities is key to effective survivorship care. In particular, knowing who is responsible for monitoring tests for recurrence (CEA, CT scanning and colonoscopy) is essential.
In particular circumstances, other models of post-treatment care can be safely and effectively provided such as nurse-led models of care (Monterosso et al. 2019). Other models of post-treatment care can be provided in these locations or by these health professionals:
- in a shared care setting
- in a general practice setting
- by non-medical staff
- by allied health or nurses
- in a non-face-to-face setting (e.g. by telehealth).
A designated member of the team should document the agreed survivorship care plan. The survivorship care plan should support wellness and have a strong emphasis on healthy lifestyle changes such as a balanced diet, a non-sedentary lifestyle, smoking cessation and alcohol reduction/abstinence, weight management and a mix of aerobic and resistance exercise (COSA 2018; Hayes et al. 2019).
This survivorship care plan should also cover, but is not limited to:
- what medical follow-up is required (surveillance for recurrence or secondary and metachronous cancers, screening and assessment for medical and psychosocial effects)
- model of post-treatment care, the health professional providing care and where it will be delivered
- care plans from other health providers to manage the consequences of cancer and cancer treatment
- wellbeing, primary and secondary prevention health recommendations that align with chronic disease management principles
- rehabilitation recommendations
- available support services
- a process for rapid re-entry to specialist medical services for suspected recurrence.
Survivors generally need regular follow-up, often for five or more years after cancer treatment finishes. The survivorship care plan therefore may need to be updated to reflect changes in the patient’s clinical and psychosocial status and needs.
Processes for rapid re-entry to hospital care should be documented and communicated to the patient and relevant stakeholders.
Care in the post-treatment phase is driven by predicted risks (e.g. the risk of recurrence, developing late effects of treatment and psychological issues) as well as individual clinical and supportive care needs. It is important that post-treatment care is based on evidence and is consistent with guidelines. Not all people will require ongoing tests or clinical review and may be discharged to general practice follow-up.
The lead clinician should discuss (and general practitioner reinforce) options for follow-up at the start and end of treatment. It is critical for optimal aftercare that the designated member of the treatment team educates the patient about the symptoms of recurrence.
General practitioners (including nurses) can:
- connect patients to local community services and programs
- manage long-term and late effects
- manage comorbidities
- provide wellbeing information and advice to promote self-management
- screen for cancer and non-cancerous conditions.
Templates and other resources to help with developing treatment summaries and survivorship care plans are available from these organisations:
- Australian Cancer Survivorship Centre
- Cancer Australia – Principles of Cancer Survivorship
- Cancer Council Australia and states and territories
- Clinical Oncology Society of Australia – Model of Survivorship Care
- eviQ – Cancer survivorship: introductory course
- MyCarePlan.org.au
- South Australian Cancer Service – Statewide Survivorship Framework resources
- American Society of Clinical Oncology – guidelines.
Not smoking, eating a healthy diet, being sun smart, avoiding or limiting alcohol intake, being physically active and maintaining a healthy body weight may help reduce the risk of primary recurrence or a second primary cancer.
Encourage and support all cancer survivors to reduce modifiable risk factors for recurrence as well as other chronic diseases. Ongoing coordination of care between providers should also deal with any comorbidities, particularly ongoing complex and life-threatening comorbid conditions.
Support cancer survivors to participate in research or clinical trials where they are available and appropriate. These might include studies to understand survivors’ issues, to better manage treatment side effects, or to improve models of care and quality of life.
For more information visit the Cancer Australia website.
See validated screening tools mentioned in Principle 4 ‘Supportive care’. Additionally, the ‘Cancer Survivors Unmet Needs (CaSun)’ is another validated screening tool that may help health professionals to identify the unmet needs of patients during survivorship.
A number of specific challenges and needs may arise for cancer survivors:
- emotional distress arising from fear of disease recurrence, returning to work, anxiety/depression, interpersonal problems and sexuality concerns
- chemotherapy-induced peripheral sensory neuropathy (may need ongoing monitoring)
- malnutrition post-treatment due to ongoing treatment side effects (e.g. weight loss or reduced oral intake) – this requires monitoring and nutrition intervention where indicated
- altered bowel function and incontinence
- stoma management
- decline in mobility or functional status as a result of treatment
- a need for increased community supports as patients recover from treatment
- financial and employment issues (e.g. loss of income and assistance with returning to work and the cost of treatment, travel and accommodation)
- appointing a substitute decision-maker and completing an advance care directive
- legal issues such as completing a will.
For more information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
Rehabilitation may be required at any point of the care pathway from the pre-treatment phase through to disease-free survival and palliative care (Cormie et al. 2017).
Issues that may need to be dealt with include managing cancer-related fatigue, coping with cognitive changes, improving physical endurance, achieving independence in daily tasks, returning to study or work and ongoing adjustment to cancer and its sequels.
Exercise is a safe and effective intervention that improves the physical and emotional health and wellbeing of cancer patients. Exercise should be embedded as part of standard practice in cancer care and be viewed as an adjunct therapy that helps counteract the adverse effects of cancer and its treatment.
Cancer survivors may find referral to specific cancer rehabilitation, optimisation programs or community-based rehabilitation appropriate and beneficial. Other options include referral to allied health supports through team care arrangements and mental health plans. Some community support organisations (cancer-related non-government, not-for-profit and charities) provide services to cancer survivors.
The lead clinician (themselves or by delegation) should take responsibility for these tasks:
- explaining the model of post-treatment care and the roles of health professionals involved in post-treatment care including the role of general practice
- explaining the treatment summary and follow-up care plan
- discussing the development of a shared follow-up and survivorship care plan where a model of shared follow-up care has been agreed
- discussing how to manage any of the physical, psychological or emotional issues identified
- providing information on the signs and symptoms of recurrent disease
- providing a survivorship care plan with information on secondary prevention and healthy living
- providing contact details of the care team involved
- providing clear information about the role and benefits of palliative care and advance care planning.
The lead clinician should ensure regular, timely, two-way communication with the general practitioner about:
- the patient’s progress
- the follow-up care plan
- potential late effects
- supportive and palliative care requirements
- any shared care arrangements
- clarification of various roles in patient care
- a process for rapid re-entry to medical services for patients with suspected recurrence or if there are other concerns.
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
Patients who present with recurrent, residual or metastatic disease should be managed by a multidisciplinary team and offered timely referral to appropriate physical, practical and emotional support.
Step 6 is concerned with managing recurrent or local residual and metastatic disease. The likelihood of recurrence depends on many factors usually related to the type of cancer, the stage of cancer at presentation and the effectiveness of treatment. Some cancers cannot be eradicated even with the best initial treatment. But controlling disease and disease-related symptoms is often possible, depending on the clinical situation.
Some patients will have recurrent disease on initial presentation. Others may present with symptoms of recurrent disease after a previous cancer diagnosis. Access to the best available therapies, including clinical trials, as well as treatment overseen by a multidisciplinary team, are crucial to achieving the best outcomes for anyone with recurrent disease.
Signs and symptoms will depend on the type of cancer initially diagnosed and the location of recurrent disease. They may be discovered by the patient or by surveillance in the post-treatment period. Recurrence may be asymptomatic or have non-specific symptoms. It should be detected by routine surveillance protocols that are applied to people who have colorectal cancer.
Managing recurrent disease is complex and should therefore involve all the appropriate specialties in a multidisciplinary team including palliative care where appropriate. From the time of diagnosis, the team should offer patients appropriate psychosocial care, supportive care, advance care planning and symptom-related interventions as part of their routine care. The approach should be personalised to meet the patient’s individual needs, values and preferences. The full complement of supportive care measures as described throughout the optimal care pathway and in Appendices A and B, and in the special population groups section should be offered to assist patients and their families and carers to cope. These measures should be updated as the patient’s circumstances change.
Survivorship care should be considered and offered at an early stage. Many people live with advanced cancer for many months or years. As survival is improving in many patients, survivorship issues should be considered as part of routine care. Health professionals should therefore be ready to change and adapt treatment strategies according to disease status, prior treatment tolerance and toxicities and the patient’s quality of life, in addition to the patient’s priorities and life plans.
If there is an indication that a patient’s cancer has returned, care should be provided under the guidance of a treating specialist. Each patient should be evaluated to determine if referral to the original multidisciplinary team is necessary. Often referral back to the original multidisciplinary team will not be necessary unless there are obvious aspects of care involving different therapeutic and supportive care disciplines not otherwise accessible. The multidisciplinary team may include new members such as palliative care specialists.
Treatment will depend on the location, extent of recurrent or residual disease, previous management and the patient’s preferences.
In managing people with colorectal cancer, treatment may include these options:
- surgery
- radiation therapy (including SABR)
- drug therapy (including chemotherapy and targeted therapy)
- locoregional therapies (including focal ablation and transarterial chemoembolisation).
In managing people with colorectal cancer, treatment programs have recently become more complex, with more effective chemotherapy and major surgical programs being offered to patients with potentially curable recurrent cancer including immunotherapy for microsatellite unstable colorectal cancers; however, therapy may focus on disease control or palliation, based on the extent of disease and the patient’s general health, preferences and values.
The potential goals of treatment should be discussed, respecting the patient’s cultural values. Wherever possible, written information should be provided.
Encourage early referral to clinical trials or accepting an invitation to participate in research.
Advance care planning is important for all patients with a cancer diagnosis but especially those with advanced disease. Patients should be encouraged to think and talk about their healthcare values and preferences with family or carers, appoint a substitute decision-maker and consider developing an advance care directive to convey their preferences for future health care in the event they become unable to communicate their wishes (AHMAC 2011).
Refer to section 4.3 ‘More information’ for links to resources.
Refer patients and carers to Advance Care Planning Australia or to the Advance Care Planning National Phone Advisory Service on 1300 208 582.
Early referral to palliative care can improve the quality of life for people with cancer and in some cases may be associated with survival benefits (Haines 2011; Temel et al. 2010; Zimmermann et al. 2014). The treatment team should emphasise the value of palliative care in improving symptom management and quality of life to patients and their carers. Refer to section 4.3 for more detailed information.
The lead clinician should ensure timely and appropriate referral to palliative care services. Referral to palliative care services should be based on the patient’s need and potential for benefit, not prognosis.
Refer to the end of section 4.3 ‘Palliative care’ for links to resources.
The treatment team should support the patient to participate in research and clinical trials where available and appropriate.
For more information visit the Cancer Australia website.
See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific challenges and needs may arise at this time for patients:
- assistance for dealing with emotional and psychological distress resulting from fear of death or dying, existential concerns, anticipatory grief, communicating wishes to loved ones, interpersonal problems and sexuality concerns
- potential isolation from normal support networks, particularly for rural patients who are staying away from home for treatment
- cognitive changes as a result of treatment and disease progression such as altered memory, attention and concentration (a patient may appoint someone to make medical, financial and legal decisions on their behalf – a substitute decision-maker – before and in case they experience cognitive decline)
- management of physical symptoms including altered bowel function and incontinence
- stoma management
- decline in mobility or functional status as a result of recurrent disease and treatments (referral to physiotherapy or occupational therapy may be required)
- coping with hair loss and changes in physical appearance (refer to the Look Good, Feel Better program– see ’Resource List’)
- appointing a substitute decision-maker and completing an advance care directive
- financial issues as a result of disease recurrence such as gaining early access to superannuation and insurance
- legal issues (completing a will, care of dependent children) and making an insurance, superannuation or social security claim on the basis of terminal illness or permanent disability.
Rehabilitation may be required at any point of the metastatic care pathway, from preparing for treatment through to palliative care. Issues that may need to be dealt with include managing cancer-related fatigue, improving physical endurance, achieving independence in daily tasks, returning to work and ongoing adjustment to cancer and its sequels.
Exercise is a safe and effective intervention that improves the physical and emotional health and wellbeing of cancer patients. Exercise should be embedded as part of standard practice in cancer care and be viewed as an adjunct therapy that helps counteract the adverse effects of cancer and its treatment.
The lead clinician should ensure there is adequate discussion with patients and carers about the diagnosis and recommended treatment, including treatment intent and possible outcomes, likely adverse effects and the supportive care options available.
Refer to Principle 6 ‘Communication’ for communication skills training programs and resources.
Step 7 is concerned with maintaining the patient’s quality of life and meeting their health and supportive care needs as they approach the end of life, as well as the needs of their family and carers.
Some patients with advanced cancer will reach a time when active treatment is no longer appropriate. The team needs to share the principles of a palliative approach to care when making decisions with the patient and their family or carer. End-of-life care is appropriate when the patient’s symptoms are increasing and functional status is declining.
If the treatment team does not include a palliative care member, the lead clinician should consider referring the patient to palliative care services, with the general practitioner’s engagement. This may include inpatient palliative unit access (as required).
The multidisciplinary team may consider seeking additional expertise from these professionals:
- clinical psychologist
- clinical nurse specialist or practitioner
- social worker
- palliative medicine specialist
- pain specialist
- pastoral or spiritual carer
- bereavement counsellor
- music therapist
- art therapist
- cultural expert
- Canteen for children of parents with cancer
The team might also recommend that patients access these services:
- home and community-based care
- specialist community palliative care workers
- community nursing.
If the patient does not already have an advance care directive in place, a designated member of the treatment team should encourage them to develop one in collaboration with their family or carer (AHMAC 2011).
It is essential for the treatment team to consider the appropriate place of care, the patient’s preferred place of death and the support needed for the patient, their family and carers.
The treatment team should also ensure that carers and families receive the information, support and guidance about their role according to their needs and wishes (Palliative Care Australia 2018).
The treatment team can refer patients and carers to these resources:
- Palliative Care Australia
- Advance Care Planning Australia or to Advance Care Planning Australia’s National Advisory Service on 1300 208 582
Clinical trials may help improve palliative care and in managing a patient’s symptoms of advanced cancer (Cancer Council Victoria 2019). The treatment team should support the patient to participate in research and clinical trials where available and appropriate.
For more information visit the Cancer Australia website. See ’Resource list’ for additional clinical trial databases.
See validated screening tools mentioned in Principle 4 ‘Supportive care’.
A number of specific challenges and needs may arise for patients at this time:
- assistance for dealing with emotional and psychological distress from anticipatory grief, fear of death or dying, anxiety/depression and interpersonal problems
- management of physical symptoms including altered bowel function and incontinence
- stoma management
- decline in mobility or functional status, affecting the patient’s discharge destination (a referral to physiotherapy, exercise physiology, occupational therapy or social work may be needed)
- appointing a substitute decision-maker and completing an advance care directive
- legal issues (completing a will, care of dependent children) and making an insurance, superannuation or social security claim on the basis of terminal illness or permanent disability
- specific support for families where a parent is dying and will leave behind bereaved children or adolescents, creating special family needs
- arranging a funeral.
These services and resources can help:
- referral to 13 11 20 for Cancer Council Australia’s Pro Bono Program for free legal, financial, small business accounting and workplace assistance (subject to a means test)
- Sad news sorry business (Queensland Health 2015) for the specific needs of Aboriginal and Torres Strait Islander people
For more information on supportive care and needs that may arise for different population groups, see Appendices A and B, and special population groups.
The lead clinician is responsible for:
- being open to and encouraging discussion with the patient about the expected disease course, considering the patient’s personal and cultural beliefs and expectations
- discussing palliative care options, including inpatient and community-based services as well as dying at home and subsequent arrangements
- providing the patient and carer with the contact details of a palliative care service
- referring the patient to palliative care in the community according to the carer’s wishes.
The lead clinician should discuss end-of-life care planning to ensure the patient’s needs and goals are met in the appropriate environment. The patient’s general practitioner should be kept fully informed and involved in major developments in the patient’s illness path.
For support with communication skills and training programs, see these sources:
The burden of cancer is not evenly spread across Australia. People experiencing socioeconomic disadvantage, Aboriginal and Torres Strait Islander communities, culturally diverse communities, people living with a disability, people with chronic mental health or psychiatric concerns and those who live in regional and rural areas of Australia have poorer cancer outcomes.
Cancer is the third leading cause of burden of disease for Aboriginal and Torres Strait Islander people. While Australia’s cancer survival rates are among the best in the world, Aboriginal and Torres Strait Islander people continue to experience a different pattern of cancer incidence and significant disparities in cancer outcomes compared with non-Indigenous Australians.
There has been a significant increase in the colorectal cancer incidence rate for Aboriginal and Torres Strait Islander people (AIHW 2018c). Between 2007 and 2014, Aboriginal and Torres Strait Islander people diagnosed with colorectal cancer had a 58 per cent chance (on average) of surviving compared with non-Indigenous Australians, who had a 67 per cent chance (on average) of surviving (AIHW 2018c).
For Aboriginal and Torres Strait Islander people, health and connection to land, culture, community and identity are intrinsically linked. Health encompasses a whole-of-life view and includes a cyclical concept of life–death–life.
The distinct epidemiology of cancer among Aboriginal and Torres Strait Islander people, and unique connection to culture, highlight the need for a specific optimal care pathway for Aboriginal and Torres Strait Islander people with cancer. Ensuring this pathway is culturally safe and supportive is vital to tackling the disparities for Aboriginal and Torres Strait Islander people.
Published in 2018, the Optimal care pathway for Aboriginal and Torres Strait Islander people with cancer provides guidance to health practitioners and service planners on optimal care for Aboriginal and Torres Strait Islander people with cancer across the cancer continuum.
In addition to the key principles underpinning cancer-specific pathways, these are the key concepts that are fundamental to Aboriginal and Torres Strait Islander health:
- providing a holistic approach to health and wellbeing
- providing a culturally appropriate and culturally safe service
- acknowledging the diversity of Aboriginal and Torres Strait Islander peoples
- understanding the social determinants and cultural determinants of health (Cancer Australia 2015).
To view the Optimal care pathway for Aboriginal and Torres Strait Islander people with cancer, visit the Cancer Australia website. To view the consumer resources – Checking for cancer and Cancer, visit the Cancer Australia website.
For people from culturally diverse backgrounds in Australia, a cancer diagnosis can come with additional complexities, particularly when English proficiency is poor. In many languages there is not a direct translation of the word ‘cancer’, which can make communicating vital information difficult. Perceptions of cancer and related issues can differ greatly in people from culturally diverse backgrounds and this can affect their understanding and decision making after a cancer diagnosis. In addition to different cultural beliefs, when English language is limited there is potential for miscommunication of important information and advice, which can lead to increased stress and anxiety for patients.
A professionally trained interpreter (not a family member or friend) should be made available when communicating with people with limited English proficiency. Navigation of the Australian healthcare system can pose problems for those with a non-Anglo culture, and members of the treatment teams should pay particular attention to supporting these patients.
The Australian Cancer Survivorship Centre has developed a glossary of more than 700 cancer terms in nine different languages. The multilingual glossary has been designed as a resource for professional translators, interpreters and bilingual health professionals working in the cancer field. The glossary is a unique tool that enables language professionals with access to accurate, consistent and culturally appropriate terminology.
Visit the Peter Mac website to see the glossary.
Disability, which can be physical, intellectual or psychological, may have existed before the cancer diagnosis or may be new in onset (occurring due to the cancer treatment or incidentally). Adjusting to life with a disability adds another challenge to cancer care and survivorship.
Several barriers prevent people with disabilities from accessing timely and effective health care (AIHW 2017):
- physical limitations
- competing health needs
- the trauma of undergoing invasive procedures
- potential barriers associated with obtaining informed consent
- failure to provide assistance with communication
- lack of information
- discriminatory attitudes among healthcare staff.
In caring for people with disabilities and a cancer diagnosis, the Australian Institute of Health and Welfare disability flag should be used at the point of admittance to correctly identify and meet the additional requirements of a person with disability. Facilities should actively consider access requirements, and health practitioners should make reasonable adjustments where required.
Patients aged between seven and 65 years who have a permanent or significant disability may be eligible for support or funding through the National Disability Insurance Scheme (National Disability Insurance Agency 2018). More information can be found on the NDIS website.
Patients aged 65 years or older (50 years or older for Aboriginal or Torres Strait Islander people) may be eligible for subsidised support and services through aged care services. An application to determine eligibility can be completed online or over the phone. More information can be found at the My Aged Care website.
‘Talking End of Life’ is a resource that shows how to teach people with intellectual disability about end of life. It is designed for disability support workers but is also helpful for others including families, health professionals and educators.
To view the resource, visit the Talking End of Life website.
Planning and delivering appropriate cancer care for older people can present a number of challenges. This could also be true for frail people or those experiencing comorbidities. Effective communication between oncology and geriatrics departments will help facilitate best practice care, which takes into account physiological age, complex comorbidities, risk of adverse events and drug interactions, as well as the implications of cognitive impairment on suitability of treatment and consent (Steer et al. 2009).
At a national interdisciplinary workshop convened by the Clinical Oncology Society of Australia, it was recommended that people over the age of 70 undergo some form of geriatric assessment, in line with international guidelines (COSA 2013; palliAGED 2018). Screening tools can be used to identify those patients in need of a comprehensive geriatric assessment (Decoster et al. 2015). This assessment can be used to help determine life expectancy and treatment tolerance and guide appropriate referral for multidisciplinary intervention that may improve outcomes (Wildiers et al. 2014).
Frailty is not captured through traditional measures of performance status (e.g. ECOG) and includes assessment in the domains of:
- function
- comorbidity
- presence of geriatric syndromes
- nutrition
- polypharmacy
- cognition
- emotional status
- social supports.
In recent years, adolescent and young adult oncology has emerged as a distinct field due to lack of progress in survival and quality-of-life outcomes (Ferrari et al. 2010; Smith et al. 2013). The significant developmental change that occurs during this life stage complicates a diagnosis of cancer, often leading to unique physical, social and emotional effects for young people at the time of diagnosis and throughout the cancer journey (Smith et al. 2012).
In caring for young people with cancer, akin to the comorbidities that require specific care in the older cancer population, the treatment team needs to pay careful attention to promoting normal development (COSA 2014). This requires personalised assessments and management involving a multidisciplinary, disease-specific, developmentally targeted approach that adheres to the following principles:
- understanding the developmental stages of adolescence and supporting normal adolescent health and development alongside cancer management
- understanding and supporting the rights of young people
- communication skills and information delivery that are appropriate to the young person
- meeting the needs of all involved, including the young person, their carers and their family
- working with educational institutions and workplaces
- considering survivorship and palliative care needs
- understanding the possibility of an inheritable basis for the person’s cancer.
An oncology team caring for an adolescent or young adult with cancer should be able to demonstrate these specific areas of expertise:
- be able to ensure access to expert adolescent and young adult health providers who have knowledge specific to the biomedical and psychosocial needs of the population
- understand the biology and current management of the disease in the adolescent and young adult age group
- consider participating in research and clinical trials for each patient
- engage in proactive discussion and management of fertility preservation, late effects of treatment, ongoing need for contraception, and psychosocial and psychosexual needs
- provide treatment in an environment that is friendly to adolescents and young adults
- consider referral to a familial cancer service.
In general, people from lower socioeconomic groups are at greater risk of poor health, have higher rates of illness, disability and death, and live shorter lives than those from higher socioeconomic groups (AIHW 2016). People experiencing socioeconomic disadvantage are less likely to participate in screening programs, more likely to be obese, less likely to exercise and much more likely to smoke, which are all risk factors for cancer. In 2010–2014 age-standardised cancer incidence rates were higher in the lowest socioeconomic areas compared with the highest socioeconomic areas for all cancers combined (Cancer Australia 2019c).
Socioeconomic status and low health literacy are closely correlated. Therefore, effective communication with patients and carers is particularly important given the prevalence of low health literacy in Australia (estimated at 60 per cent of Australian adults) (ACSQHC 2014).
Consideration should be taken for cancer patients experiencing socioeconomic disadvantage to reduce their risk of being underserved for health care.
A diagnosis of cancer may present additional challenges to people who have pre-existing chronic mental health or psychiatric concerns, resulting in exacerbation of their mental health symptoms. This may include heightened anxiety, worsening depression or thoughts of self-harm.
As poor adjustment and coping can affect treatment decisions, people who are known to have a mental health diagnosis need psychosocial assessment in the oncology setting to formulate a plan for ongoing support throughout treatment.
Psychosocial support can assist with challenges in communicating with health professionals, enhance understanding of the treatment journey, ensure capacity for consent to treatment options and improve compliance with treatment requests. A referral for psychosocial support from a health professional to the psycho-oncology team can ensure these patients are provided with targeted interventions or referrals to community-based services that may mitigate problems associated with the impacts of social isolation that frequently accompany chronic mental ill-health.
Many patients with chronic mental health problems may be well known to external service providers. Psycho-oncology health professionals can form meaningful partnerships with existing service providers to optimise patient care throughout treatment and beyond.
Drug use disorders fall within the area of mental health conditions. People who are opiate dependent may have specific and individual requirements regarding pain management and their own preference for type of opiate prescribed or used.
People who identify as sexually or gender diverse may have unique needs following a cancer diagnosis. Sexually or gender diverse identities include (but are not limited to) people who identify as lesbian, gay, bisexual or transgender, collectively ‘LGBT’. There is no universally agreed upon initialism to describe this community, with other terms such as queer/questioning (Q), intersex (I), asexual (A) and pansexual (P) often included, as well as a plus symbol (+) indicating inclusivity of other identities not explicitly mentioned.
Sexual orientation and gender identity are relevant across the entire spectrum of cancer care, from prevention to survivorship and end-of-life care. LGBT people are less likely to participate in cancer screening, and some segments of the LGBT community exhibit elevated rates of specific cancer risk factors – for example, higher rates of smoking and alcohol use. Regarding treatment, there may be unique factors relevant to LGBT people that may affect decision making. Additionally, the LGBT population experiences higher rates of anxiety, depression and stressful life circumstances, and may be at risk of inferior psychosocial outcomes following a cancer diagnosis. LGBT people are also more likely to be estranged from their families of origin, and for older people, less likely to have adult children who may provide support and care.
Barriers to care for LGBT people include past negative interactions with healthcare systems, experiences or fear of discrimination and harassment in healthcare settings, assumptions of cisgender/heterosexual identity, lack of recognition or exclusion of same-sex partners from care, and a lack of relevant supportive care and information resources.
Some LGBT patients may have issues peculiar to their sexual orientation in the context of colorectal cancer and its treatment (e.g. after proctectomy) that need sensitive handling.
To provide safe and appropriate care for LGBT people with cancer, healthcare providers should:
- display environmental cues to show an inclusive and safe setting for LGBT patients
- avoid assumptions about the sexual orientation or gender identity of patients and their partners
- facilitate positive disclosure of sexual orientation or gender identity
- include same-sex/gender partners and families of choice in care
- be aware of relevant supportive care and information resources
- provide non-judgemental, patient-centred care.
Supportive care in cancer refers to the following five domains:
- the physical domain, which includes a wide range of physical symptoms that may be acute, relatively short lived or ongoing, requiring continuing interventions or rehabilitation
- the psychological domain, which includes a range of issues related to the patient’s mental health wellbeing and personal relationships
- the social domain, which includes a range of social and practical issues that will affect the patient, carer and family such as the need for emotional support, maintaining social networks and financial concerns
- the information domain, which includes access to information about cancer and its treatment, recovery and survivorship support services and the health system overall
- the spiritual domain, which focuses on the patient’s changing sense of self and challenges to their underlying beliefs and existential concerns (Palliative Care Victoria 2019).
Fitch’s (2000) model of supportive care recognises the variety and level of intervention required at each critical point as well as the need to be specific to the individual patient. The model targets the type and level of intervention required to meet patients’ supportive care needs.
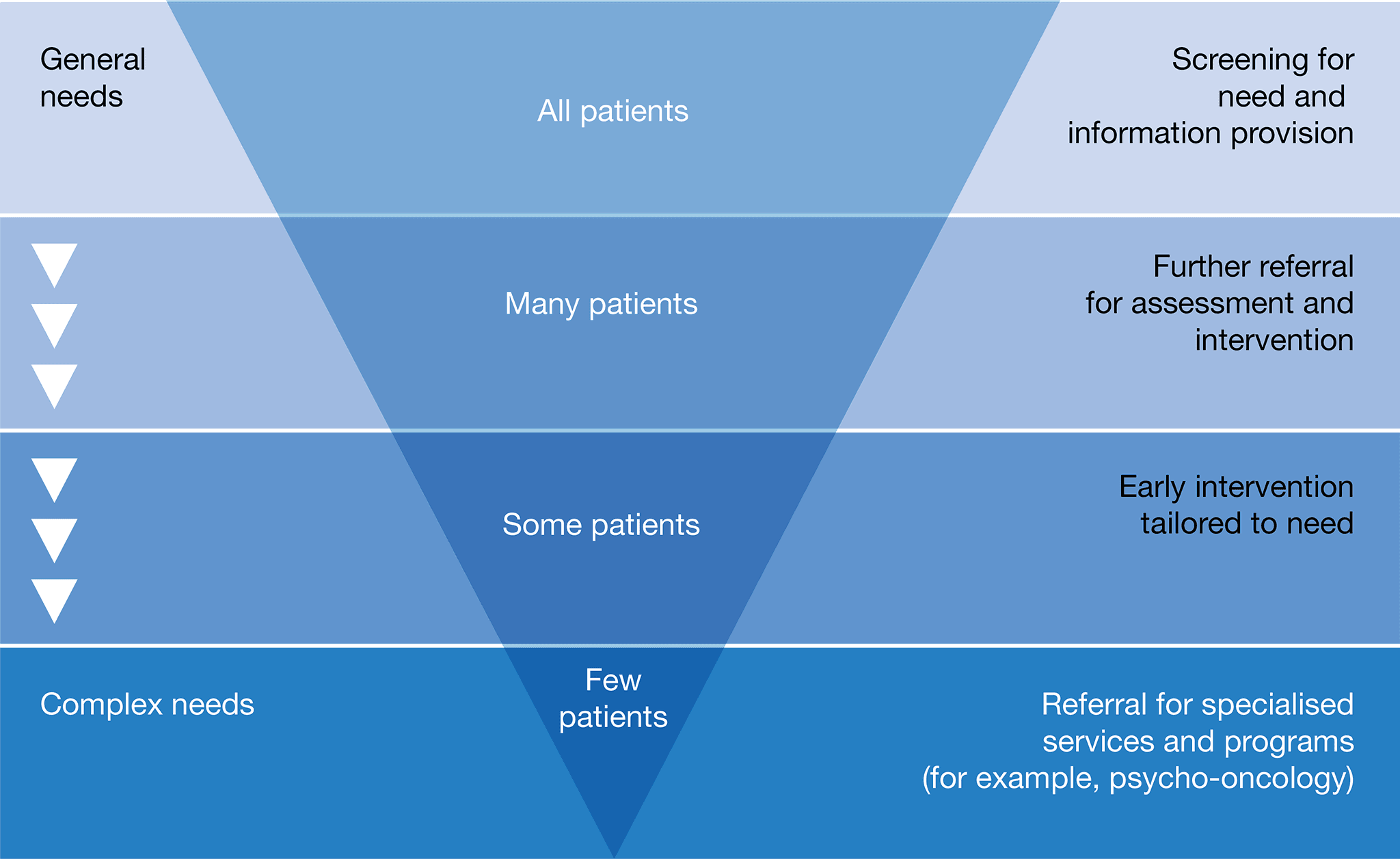
Consider a referral to a psychologist, psychiatrist, pastoral/spiritual care practitioner, social worker, specialist nurse or a relevant community-based program if the patient has these issues:
- displaying emotional cues such as tearfulness, distress that requires specialist intervention, avoidance or withdrawal
- being preoccupied with or dwelling on thoughts about cancer and death
- displaying fears about the treatment process or the changed goals of their treatment
- displaying excessive fears about cancer progression or recurrence
- worrying about loss associated with their daily function, dependence on others and loss of dignity
- becoming isolated from family and friends and withdrawing from company and activities that they previously enjoyed
- feeling hopeless and helpless about the effect that cancer is having on their life and the disruption to their life plans
- struggling to communicate with family and loved ones about the implications of their cancer diagnosis and treatment
- experiencing changes in sexual intimacy, libido and function
- struggling with the diagnosis of metastatic or advanced disease
- having difficulties quitting smoking (refer to Quitline on 13 7848) or with other drug and alcohol use
- having difficulties transitioning to palliative care.
Additional considerations that may arise for the multidisciplinary team include:
- support for the carer – encourage referrals to psychosocial support from a social worker, psychologist or general practitioner
- referral to an exercise physiologist or physiotherapist as a therapeutic approach to prevent and manage psychological health
- referral to wellness-after-cancer programs to provide support, information and offer strategies.
Complementary therapies may be used together with conventional medical treatments to support and enhance quality of life and wellbeing. They do not aim to cure the patient’s cancer. Instead, they are used to help control symptoms such as pain and fatigue (Cancer Council Australia 2019).
The lead clinician or health professional involved in the patient’s care should discuss the patient’s use (or intended use) of complementary therapies not prescribed by the multidisciplinary team to assess safety and efficacy and to identify any potential toxicity or drug interactions.
The lead clinician should seek a comprehensive list of all complementary and alternative medicines being taken and explore the patient’s reason for using these therapies and the evidence base. A transparent and honest discussion that is free from judgement should be encouraged.
While some complementary therapies are supported by strong evidence, others are not. For such therapies, the lead clinician should discuss their potential benefits and use them alongside conventional therapies (NHMRC 2014).
If the patient expresses an interest in using complementary therapies, the lead clinician should consider referring patients to health providers within the multidisciplinary team who have expertise in the field of complementary and alternative therapies (e.g. a clinical pharmacist, dietitian or psychologist) to assist them to reach an informed decision. Costs of such approaches should be part of the discussion with the patient and considered in the context of evidence of benefit.
The lead clinician should assure patients who use complementary therapies that they can still access a multidisciplinary team review and encourage full disclosure about therapies being used.
- See Cancer Australia’s position statement on complementary and alternative therapies.
- See the Clinical Oncological Society of Australia’s position statement Use of complementary and alternative medicine by cancer patients .
Advance Care Planning Australia
Advance Care Planning Australia provides national advance care planning resources for individuals, families, health professional and service providers. Resources include a national advisory service, information resources, a legal forms hub and education modules.
- Telephone: 1300 208 582
- Website
Australian Cancer Survivorship Centre
The Australian Cancer Survivorship Centre has developed information resources and events to help people move from initial treatment to post treatment and beyond, including those receiving maintenance treatments. While they do not provide clinical advice, they connect with a range of providers to enable improved care.
- Telephone: (03) 8559 6220
- Website
Australian Commission on Safety and Quality in Health Care
The Australian Commission on Safety and Quality in Health Care has developed a resource for patients and carers explaining the coordination of care that patients should receive from their health service during cancer treatment. The resource is called What to expect when receiving medication for cancer care.
Beyond Blue
Beyond Blue provides information about depression, anxiety and related disorders, as well as about available treatment and support services.
- Telephone: 1300 22 4636
- Website
Bowel Cancer Australia
Bowel Cancer Australia provides information about bowel cancer prevention, diagnosis and treatment.
- Telephone: 1800 555 494
- Website
Cancer Council Australia – Bowel cancer
Cancer Council Australia provides information on The National Bowel Cancer Screening Program, including eligibility to receive a test.
- Telephone: 1300 22 4636
- Website
Cancer Australia
Cancer Australia provides information for consumers, carers and their families including printed resources and video content.
Cancer Council’s Cancer Information and Support Service
Cancer Council 13 11 20 is a confidential telephone support service available to anyone affected by cancer. This service acts as a gateway to evidence-based documented, practical and emotional support available through Cancer Council services and other community organisations. Calls will be answered by a nurse or other oncology professional who can provide information relevant to a patient’s or carer’s situation. Health professionals can also access this service.
- Telephone: 13 11 20 – Monday to Friday, 9.00am to 5.00pm (some states have extended hours)
- Website
Cancer Council’s Cancer Connect
Cancer Connect is a free and confidential telephone peer support service that connects someone who has cancer with a specially trained volunteer who has had a similar cancer experience.
A Connect volunteer can listen with understanding and share their experiences and ways of coping. They can provide practical information, emotional support and hope. Many people newly diagnosed with cancer find this one-to-one support very beneficial.
For more information on Cancer Connect call Cancer Council on 13 11 20.
Canteen
Canteen helps adolescents, young adults and parents to cope with cancer in their family. Canteen offers individual support services, peer support services and a youth cancer service, as well as books, resources and useful links.
- Telephone: 1800 835 932 to talk to a health professional about information and support for young people or 1800 226 833 for other enquiries
- Website
Care Search: Palliative Care Knowledge Network
Care Search provides information for patients and carers on living with illness and how to find services. Practical advice on how to care is also available.
- Telephone: (08) 7221 8233
- Website
Clinical trial information
For a collection of clinical trials available in Australia see the following sources of information:
CanEAT pathway
A guide to optimal cancer nutrition for people with cancer, carers and health professionals.
Guides to best cancer care
The short guides help patients, carers and families understand the optimal cancer care that should be provided at each step. They include optimal timeframes within which tests or procedures should be completed, prompt lists to support patients to understand what might happen at each step of their cancer journey and to consider what questions to ask, and provide information to help patients and carers communicate with health professionals.
The guides are located on an interactive web portal, with downloadable PDFs available in multiple languages.
Look Good, Feel Better
A free national community service program, run by the Cancer Patients Foundation, dedicated to teaching cancer patients how to manage the appearance-related side effects caused by treatment for any type of cancer.
- Telephone: 1800 650 960
- Website
National Bowel Cancer Screening Program
Find information about the National Bowel Screening Program.
- Program Information Line: 1800 118 868
- Test Kit Helpline: 1800 930 998
- Translating & Interpreting Service: 13 14 50
- Website
Quitline
Quitline is a confidential, evidence-based telephone counselling service. Highly trained Quitline counsellors use behaviour change techniques and motivational interviewing over multiple calls to help people plan, make and sustain a quit attempt.
Quitline is a culturally inclusive service for all, and Aboriginal counsellors are also available. Health professionals can refer patients to Quitline online or via fax.
- Telephone: 13 7848
- Website or the relevant website in your state or territory.
Australian Cancer Survivorship Centre
The Australian Cancer Survivorship Centre provides expertise in survivorship care, information, support and education. Its purpose is to support and enable optimal survivorship care.
- Telephone: (03) 8559 6220
- Website
Australian Commission on Safety and Quality in Health Care
The Australian Commission on Safety and Quality in Health Care has developed a guide for clinicians containing evidence-based strategies to support clinicians to understand and fulfil their responsibilities to cancer patients. This guide is particularly relevant to Steps 3 to 6 of the optimal care pathway. The guide is titled NSQHS Standards user guide for medication management in cancer care for clinicians.
Cancer Australia
Information for health providers including guidelines, cancer learnings, cancer guides, reports, resources, videos, posters and pamphlets.
Cancer Council Australia
Information on prevention, research, treatment and support provided by Australia’s peak independent cancer authority.
CanEAT pathway
A guide to optimal cancer nutrition for people with cancer, carers and health professionals.
eviQ
A clinical information resource providing health professionals with current evidence-based, peer-maintained, best practice cancer treatment protocols and information relevant to the Australian clinical environment.
National Health and Medical Research Council
Information on clinical practice guidelines, cancer prevention and treatment.
Advance care directive – voluntary person-led document that focus on an individual’s values and preferences for future health and medical treatment decisions, preferred outcomes and care. They are completed and signed by a competent person. They are recognised by specific legislation (statutory) or common law (non-statutory). Advance care directives can also appoint the substitute decision-maker(s) who can make decisions about health or personal care on the individual’s behalf if they are no longer able to make decisions themselves. Advance care directives focus on the future health care of a person, not on the management of his or her assets. They come into effect when an individual loses decision-making capacity.
Advance care planning – the process of planning for future health and personal care, where the person’s values, beliefs and preferences are made known so they can guide decision making at a future time when that person cannot make or communicate their decisions.
Alternative therapies – treatments used in place of conventional medical treatment.
Care coordinator – the health provider nominated by the multidisciplinary team to coordinate patient care. The care coordinator may change over time depending on the patient’s stage in the care pathway and the location and care in which care is being delivered.
Castrate resistant progressive disease – progressive disease despite castrate levels of testosterone (Cancer Council Australia Advanced Prostate Cancer Guidelines Working Party 2010).
Complementary therapies – supportive treatment used in conjunction with conventional medical treatment. These treatments may improve wellbeing and quality of life and help people deal with the side effects of cancer.
End-of-life care – includes physical, spiritual and psychosocial assessment, and care and treatment, delivered by health professionals and ancillary staff. It also includes support of families and carers and care of the patient’s body after their death.
Immunotherapy – a type of cancer treatment that helps the body’s immune system to fight cancer. Immunotherapy can boost the immune system to work better against cancer or remove barriers to the immune system attacking the cancer.
Indicator – a documentable or measurable piece of information regarding a recommendation in the optimal care pathway.
Informed financial consent – the provision of cost information to patients, including notification of likely out-of-pocket expenses (gaps), by all relevant service providers, preferably in writing, before admission to hospital or treatment (Commonwealth Department of Health 2017).
Lead clinician – the clinician who is nominated as being responsible for individual patient care. The lead clinician may change over time depending on the stage of the care pathway and where care is being provided.
Metastatic disease – cancer that has spread from the part of the body where it started (the primary site) to other parts of the body.
Multidisciplinary care – an integrated team approach to health care in which medical and allied health providers consider all relevant treatment options and collaboratively develop an individual treatment plan for each patient.
Multidisciplinary team – comprises the core disciplines that are integral to providing good care. The team is flexible in approach, reflects the patient’s clinical and psychosocial needs and has processes to facilitate good communication.
Multidisciplinary team meeting – a meeting of health professionals from one or more clinical disciplines who together make decisions about recommended treatment of patients.
Optimal care pathway – the key principles and practices required at each stage of the care pathway to guide the delivery of consistent, safe, high-quality and evidence-based care for all people affected by cancer.
Palliative care – any form of medical care or treatment that concentrates on reducing the severity of disease symptoms.
Patient management frameworks – tumour stream models adopted in Victoria in 2003 to reduce variation in cancer care. The optimal care pathways are updated versions of these models.
Performance status – an objective measure of how well a patient can carry out activities of daily life.
Prehabilitation – one or more interventions performed in a newly diagnosed cancer patient that are designed to improve physical and mental health outcomes as the patient undergoes treatment and beyond.
Primary care health professional – in most cases this is a general practitioner but may also include general practice nurses, community nurses, nurse practitioners, allied health professionals, midwives, pharmacists, dentists and Aboriginal health workers.
Primary specialist – the person who makes the referral to the multidisciplinary team (such as specialist physician, surgeon, oncologist, palliative care specialist). This person will also make referrals for treatment and will be responsible for overseeing follow-up care.
PSMA PET/CT – a whole body scan that images prostate cancer wherever it is located in the body.
Rehabilitation – comprises multidisciplinary efforts to allow the patient to achieve optimal physical, social, physiological and vocational functioning within the limits imposed by the disease and its treatment.
Spiritual care – the aspect of humanity that refers to the way individuals seek and express meaning and purpose and the way they experience their connectedness to the moment, to self, to others, to nature, and to the significant or sacred.
Substitute decision-maker – a person permitted under the law to make decisions on behalf of someone who does not have competence or capacity.
Supportive care – care and support that aims to improve the quality of life of people living with cancer, cancer survivors and their family and carers and particular forms of care that supplement clinical treatment modalities.
Survivorship – an individual is considered a cancer survivor from the time of diagnosis, and throughout their life; the term includes individuals receiving initial or maintenance treatment, in recovery or in the post-treatment phase.
Survivorship care plan – a formal, written document that provides details of a person’s cancer diagnosis and treatment, potential late and long-term effects arising from the cancer and its treatment, recommended follow-up, surveillance, and strategies to remain well.
Targeted therapy – a medicine that blocks the growth and spread of cancer by interfering with specific molecules.
We acknowledge the Traditional Owners of Country throughout Australia and their continuing connection to the land, sea and community. We pay our respects to them and their cultures and to Elders past, present and emerging.
This work is available from the Cancer Council website.
First published in November 2014. Updated April 2016. This edition published in June 2021.
ISBN: 978-1-76096-144-2
Suggested citation: Cancer Council Victoria and Department of Health Victoria 2021, Optimal care pathway for people with colorectal cancer, 2nd edn, Cancer Council Victoria, Melbourne.
Enquiries about this publication can be sent to optimalcare.pathways@cancervic.org.au.
Our thanks to the following health professionals, consumer representatives, stakeholders and organisations consulted in developing this optimal care pathway.
Professor Alexander Heriot (Chair), Colorectal Surgeon, Peter MacCallum Cancer Centre, The University of Melbourne
Professor Alex Boussioutas, Gastroenterologist, The Royal Melbourne Hospital, Peter MacCallum Cancer Centre, The University of Melbourne
Professor Peter Gibbs, Medical Oncologist, Western Health
Dr Michael Homewood, General Practitioner, Lara Medical Centre
Associate Professor Sam Ngan, Radiation Oncologist, Peter MacCallum Cancer Centre
Ms Wendy Sansom, CNC Stomal Therapy Nurse, Eastern Health
Professor Robert Thomas, Special Advisor on Health, Professorial Fellow, The University of Melbourne
Ms Julia Brancato, Project Coordinator, Cancer Council Victoria
Professor Alexander Heriot, Colorectal Surgeon, Peter MacCallum Cancer Centre, The University of Melbourne (Chair)
Ms Georgina Akers, Colorectal Cancer Follow-up Nurse, Western Health
Mrs Sandra Anderson, Consumer Representative, Barwon Health
Ms Jenni Bourke, Occupational Therapist, Peter MacCallum Cancer Centre
Associate Professor Alex Boussioutas, Gastroenterologist, The Royal Melbourne Hospital, Peter MacCallum Cancer Centre, The University of Melbourne
Mrs Marilyn Dolling, Consumer Representative, Barwon Health
Dr Louise Gorman, Radiation Oncologist, Ballarat Austin Radiation Oncology Centre
Mahesh Iddawela, Consultant Medical Oncologist, Goulburn Valley Health; Senior Lecturer, RHAC, The University of Melbourne
Dr Michael Homewood, General Practitioner, Lara Medical Centre
Dr Greg Mewett, Palliative Care Physician, Ballarat Health Care
Associate Professor Sam Ngan, Radiation Oncologist, Peter MacCallum Cancer Centre, The University of Melbourne
Ms Wendy Sansom, CNC Stomal Therapy Nurse, Eastern Health
Mr Henrick Schwalb, Surgeon, Border East Clinical
Professor Robert Thomas, Chief Advisor on Cancer, Victorian Department of Health
Advance Care Planning Australia
Allied Health Professions Australia
Australasian Association of Nuclear Medicine Specialists
Australasian Chapter of Palliative Medicine, Royal Australia College of Physicians
Australasian Gastro-Intestinal Trials Group
Australian and New Zealand Society of Neuroradiology
Australian and New Zealand Society of Palliative Care
Australian Cancer Survivorship Centre
Australian College of Nursing
Australian Medical Association
Australian Society of Medical Imaging and Radiation Therapy
Cancer Nurses Society of Australia
Clinical Oncology Society of Australia
Colorectal Surgical Society of Australia and New Zealand
Dietitians Australia
Gastroenterological Society of Australia
Interventional Radiology Society of Australasia
Medical Oncology Group of Australia
Oncology Social Workers Australia and New Zealand
Royal Australasian College of Physicians
Royal Australasian College of Surgeons
Royal Australian and New Zealand College of Radiologists
Royal Australian College of General Practitioners
Royal College of Pathologists of Australasia
Alfred Health
Cancer Australia
Cancer Council Victoria, Strategy and Support
Cancer Institute New South Wales
Consumer representative
Concord Repatriation General Hospital New South Wales
Department of Health Victoria, Commissioning and System Improvement Division, Cancer Unit
National Cancer Expert Reference Group
Olivia Newton-John Cancer Wellness and Research Centre
St Vincent’s Hospital Melbourne
Other stakeholders consulted to provide feedback including relevant Cancer Council committees and networks, Integrated Cancer Services, Primary Health Networks and a number of health services.
The multidisciplinary team may include the following members:
- care coordinator (as determined by multidisciplinary team members)*
- gastroenterologist with colorectal expertise*
- general and/or colorectal surgeon*
- medical oncologist*
- nurse (with appropriate expertise)*
- pathologist*
- radiation oncologist*
- radiologist with expertise in MRI*
- stomal therapy nurse*
- Aboriginal health practitioner, Indigenous liaison officer or remote general practitioner
- clinical psychologist
- clinical trials coordinator
- dietitian
- exercise physiologist
- fertility specialist
- general practitioner
- geneticist or genetic counsellor
- hepato-pancreatobiliary surgeon
- interventional radiologist
- nuclear medicine physician with PET expertise
- occupational therapist
- palliative care specialist
- pharmacist
- physiotherapist
- psychiatrist
- social worker
- spiritual/pastoral care
- thoracic surgeon.
* Denotes core members. Core members of the multidisciplinary team are expected to attend most multidisciplinary team meetings either in person or remotely.
Australian Adult Cancer Pain Management Guideline Working Party 2019, Australian adult cancer pain management guideline: ‘Cancer pain management in adults’, Cancer Council Australia, Sydney, viewed 6 June 2019, https://wiki.cancer.org.au/australiawiki/index.php?oldid=191646.
Australian Cancer Survivorship Centre 2016, ‘Survivorship care planning’, viewed 2 October 2020, https://www.petermac.org/sites/default/files/page/downloads/Survivorship_Care_Planning.pdf.
Australian Cancer Survivorship Centre 2019, Community support organisations’ cancer survivorship care consensus statement, viewed 10 February 2020, https://www.petermac.org/sites/default/files/media-uploads/NGO_ConsensusStatement.pdf.
Australian Clinical Trials 2015, ‘Potential benefits and risks’, National Health and Medical Research Council, Department of Industry, Innovation and Science, Australian Government, Canberra, viewed 24 July 2019, https://www.australianclinicaltrials.gov.au/why-be-part-clinical-trial/potential-benefits-and-potential-risks.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2014, Health literacy: taking action to improve safety and quality, ACSQHC, Sydney, viewed 18 February 2020, https://www.safetyandquality.gov.au/sites/default/files/migrated/Health-Literacy-Taking-action-to-improve-safety-and-quality.pdf.
Australian Commission for Safety and Quality in Health Care (ACSQHC) 2015, Credentialing health practitioners and defining their scope of clinical practice: a guide for managers and practitioners, ACSQHC, Sydney, viewed 18 February 2020, https://www.safetyandquality.gov.au/publications-and-resources/resource-library/credentialing-health-practitioners-and-defining-their-scope-clinical-practice-guide-managers-and-practitioners.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2017, National Safety and Quality Health Service Standards: guide for hospitals, ACSQHC, Sydney, viewed 18 February 2020, https://www.safetyandquality.gov.au/wp-content/uploads/2017/12/National-Safety-and-Quality-Health-Service-Standards-Guide-for-Hospitals.pdf.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2019a, Person-centred care, ACSQHC, Sydney, viewed 15 May 2020, https://www.safetyandquality.gov.au/our-work/partnering-consumers/person-centred-care.
Australian Commission on Safety and Quality in Health Care (ACSQHC) 2019b, Australian Hospital Patient Experience Question Set, ACSQHC, Sydney, viewed 25 March 2020, https://www.safetyandquality.gov.au/our-work/indicators-measurement-and-reporting/australian-hospital-patient-experience-question-set.
Australian Commission for Safety and Quality in Health Care (ACSQHC) 2020, ‘NSQHS Standards user guide for medication management in cancer care’, ACSQHC, Sydney, viewed 16 April 2020, https://www.safetyandquality.gov.au/publications-and-resources/resource-library/nsqhs-standards-user-guide-medication-management-cancer-care.
Australian Health Ministers’ Advisory Council (AHMAC) 2011, A national framework for advance care directives, Council of Australian Governments Health Council, Adelaide, viewed 22 July 2019 http://www.coaghealthcouncil.gov.au/Portals/0/A%20National%20Framework%20for%20Advance%20Care%20Directives_September%202011.pdf.
Australian Institute of Health and Welfare (AIHW) 2016, Australia’s health 2016, Australia’s health series no. 15. Cat. no. AUS 199, AIHW, Canberra.
Australian Institute of Health and Welfare (AIHW) 2017, Access to health services by Australians with disability, viewed 20 February 2020, https://www.aihw.gov.au/reports/disability/access-health-services-disability/contents/content.
Australian Institute of Health and Welfare (AIHW) 2018a, Australia’s health 2018, Australia’s health series no. 16. Cat. no. AUS 221, AIHW, Canberra.
Australian Institute of Health and Welfare (AIHW) 2018b, Analysis of bowel cancer outcomes for the National Bowel Cancer Screening Program 2018, Cat. no. CAN 113, AIHW, Canberra, viewed 16 July 2020, https://www.aihw.gov.au/reports/cancer-screening/analysis-of-bowel-cancer-outcomes-nbcsp-2018/contents/table-of-contents.
Australian Institute of Health and Welfare (AIHW) 2018c, Cancer in Aboriginal & Torres Strait Islander people of Australia, Cat. no. CAN 109, AIHW, Canberra, viewed 17 July 2020, https://www.aihw.gov.au/reports/cancer/cancer-in-indigenous-australians/contents/cancer-type/colorectal-cancer-c18-c20.
Averyt, J, Nishimoto, PW 2014, ‘Addressing sexual dysfunction in colorectal cancer survivorship care’, Journal of Gastrointestinal Oncology, vol. 5, no. 5, pp. 388–394.
AYA Cancer Fertility Preservation Guidance Working Group 2014, Fertility preservation for AYAs diagnosed with cancer: guidance for health professionals, Cancer Council Australia, Sydney, viewed 20 July 2020, https://wiki.cancer.org.au/australia/COSA:AYA_cancer_fertility_preservation/Introduction.
Cancer Australia 2015, National Aboriginal and Torres Strait Islander cancer framework, Cancer Australia, Surry Hills, viewed 24 August 2017, https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/national-aboriginal-and-torres-strait-islander-cancer-framework.
Cancer Australia 2017, Principles of cancer survivorship, Australian Government, Sydney, viewed 18 February 2020, https://canceraustralia.gov.au/sites/default/files/publications/principles-cancer-survivorship/pdf/pocs_-_principles_of_cancer_survivorship.pdf.
Cancer Australia 2019a, Principles of multidisciplinary care, Australian Government, Sydney, viewed 18 July 2019, https://canceraustralia.gov.au/system/tdf/guidelines/all_about_multidisciplinary_care.pdf?file=1&type=node&id=3551.
Cancer Australia 2019b, ‘Bowel cancer (Colorectal cancer) in Australia statistics’, viewed 18 February 2020, https://bowel-cancer.canceraustralia.gov.au/statistics.
Cancer Australia 2019c, ‘Cancer incidence’, viewed 18 February 2020, https://ncci.canceraustralia.gov.au/diagnosis/cancer-incidence/cancer-incidence.
Cancer Australia 2020a, Cancer care in the time of COVID-19: a conceptual framework for the management of cancer during a pandemic, Australian Government, Sydney, viewed 3 September 2020, https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/cancer-care-time-covid-19-conceptual-framework-management-cancer-during-pandemic.
Cancer Australia 2020b, ‘Bowel cancer (Colorectal cancer) in Australia statistics’, viewed 18 February 2020, https://bowel-cancer.canceraustralia.gov.au/statistics.
Cancer Council Australia 2018, ‘Prevention’, Cancer Council Australia, Sydney, viewed 18 July 2019, https://www.cancer.org.au/preventing-cancer/.
Cancer Council Australia 2019, ‘Complementary and alternative therapies’, Cancer Council Australia, Sydney, viewed 22 July 2019, https://www.cancer.org.au/about-cancer/treatment/complementary-therapies-and-cancer.html.
Cancer Council Australia 2020, ‘National cancer control policy’, Cancer Council Australia, Sydney, viewed 17 July 2020, https://wiki.cancer.org.au/policy/Bowel_cancer/Screening.
Cancer Council Australia Colorectal Cancer Guidelines Working Party 2019, Clinical practice guidelines for the prevention, early detection and management of colorectal cancer, Cancer Council Australia, Sydney, viewed 9 December 2019 https://wiki.cancer.org.au/australia/Guidelines:Colorectal_cancer.
Cancer Council Victoria 2019, ‘Palliative care’, Cancer Council Victoria, viewed 7 October 2019, https://www.cancervic.org.au/cancer-information/treatments/treatments-types/palliative_care/palliative-care-treatment.html.
Cancer Research in Primary Care 2016, Principles statement: Shared care, PC4 Shared Care Working Group, Melbourne, viewed 4 October 2019, http://pc4tg.com.au/wp-content/uploads/2016/07/PC4-Principles-Statement-shared-care-2016-1.pdf.
Clinical Oncology Society of Australia (COSA) 2013, Special Issue: COSA’s 40th Annual Scientific Meeting, Cancer Care Coming of Age, 12–14 November 2013, Adelaide Convention Centre, Asia-Pacific Journal of Clinical Oncology, vol. 9, no. 3, pp. 61–98.
Clinical Oncology Society of Australia (COSA) 2014, Psychosocial management of AYAs diagnosed with cancer: guidance for health professionals, COSA, Sydney, viewed 7 October 2019, http://wiki.cancer.org.au/australia/COSA:Psychosocial_management_of_AYA_cancer_patients.
Clinical Oncology Society of Australia (COSA) 2015, Cancer care coordinator: position statement, COSA, Sydney, viewed 22 July 2019, https://www.cosa.org.au/media/332296/cancer-care-coordinator-position-statement_final-endorsed-by-council_161115_cnsa-logo.pdf.
Clinical Oncology Society of Australia (COSA) 2016, Australasian tele-trial model: access to clinical trials closer to home using tele-health a national guide for implementation, v1.7, COSA, Sydney, viewed 18 July 2019, https://www.cosa.org.au/media/332325/cosa-teletrial-model-final-19sep16.pdf.
Clinical Oncology Society of Australia (COSA) 2018, COSA position statement on exercise in cancer care, COSA, Sydney, viewed 22 July 2019, https://www.cosa.org.au/media/332488/cosa-position-statement-v4-web-final.pdf.
Commonwealth Department of Health 2017, Out-of-pocket expenses for private medical treatment (informed financial consent), Commonwealth of Australia, Canberra.
Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, et al. 2018, ‘Clinical Oncology Society of Australia position statement on exercise in cancer care’, Medical Journal of Australia, vol. 209, no. 4, pp. 184–187.
Cormie P, Zopf EM, Zhang X, Schmitz KH 2017, ‘The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects’, Epidemiologic Reviews, vol. 39, no. 1, pp. 71–92.
Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. 2015, ‘Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations’, Annals of Oncology, vol. 26, no.1, pp 288–300.
El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, et al. 2015, ‘American Cancer Society colorectal cancer survivorship care guidelines’, CA: A Cancer Journal for Clinicians, vol. 65, pp. 427–455.
Emery J 2014, ‘Cancer survivorship – the role of the GP’, Australian Family Physician, vol. 43, no. 8, pp. 521–525.
eviQ 2019, ‘Safe handling and waste management of hazardous drugs’, Cancer Institute NSW, Sydney, viewed 22 July 2019, https://www.eviq.org.au/clinical-resources/administration-of-antineoplastic-drugs/188-safe-handling-and-waste-management-of-hazardou.
Ferrari A, Thomas D, Franklin A, Hayes-Lattin B, Mascarin M, van der Graaf W, et al. 2010, ‘Starting an adolescent and young adult program: some success stories and some obstacles to overcome’, Journal of Clinical Oncology, vol. 28, no. 32, pp. 4850–4857.
Fitch M 2000, ‘Supportive care for cancer patients’, Hospital Quarterly, vol. 3, no. 4, pp. 39–46.
Fitch MI 2008, ‘Supportive care framework’, Canadian Oncology Nursing Journal, vol. 18, no. 1, pp. 6–14.
Gilligan T, Coyle N, Frankel RM, Berry DL, Bohlke K, Epstein, RM, et al. 2017, ‘Patient–clinician communication: American Society of Clinical Oncology consensus guideline’, Journal of Clinical Oncology, vol. 35, no. 31, pp. 3618–3623.
Hack TF, Reuther DJ, Weir LM, Grenier D, Degner LF 2012, ‘Promoting consultation recording practice in oncology: identification of critical implementation factors and determination of patient benefit’, Psycho-Oncology, vol. 22, pp. 1273–1282.
Haines IE 2011, ‘Managing patients with advanced cancer: the benefits of early referral for palliative care’, Medical Journal of Australia, vol. 194, no. 3, pp. 107–108.
Hayes SC, Newton RU, Spence RR, Galvao DA 2019, ‘The Exercise and Sports Science Australia position statement: exercise medicine in cancer management’, Journal of Science and Medicine in Sport, vol. 22, no. 11, pp. 1175–1199.
Hewitt M, Greenfield S, Stovall E 2006, From cancer patient to cancer survivor: lost in transition, National Academies Press, Washington.
Hong KS, Oh B, Kim E, Chung SS, Kim KH, Lee R 2014, ‘Psychological attitude to self-appraisal of stoma patients: prospective observation of stoma duration effect to self-appraisal’, Annals of Surgical Treatment and Research, vol. 86, no. 3, pp. 152–160.
Jefford M, Ward AC, Lisy K, Lacey K, Emery JD, Glaser AW, et al. 2017, ‘Patient-reported outcomes in cancer survivors: a population-wide cross-sectional study’, Support Care Cancer, no. 10, pp. 3171–3179.
Laidsaar-Powell R, Butow P, Boyle F, Juraskova 2018a, ‘Facilitating collaborative and effective family involvement in the cancer setting: guidelines for clinicians (TRIO guidelines-1)’, Patient Education and Counseling, vol. 101, no. 6, pp. 970–982.
Laidsaar-Powell R, Butow P, Boyle F, Juraskova 2018b, ‘Managing challenging interactions with family caregivers in the cancer setting: guidelines for clinicians (TRIO guidelines-2)’, Patient Education and Counselling, vol. 101, no. 6, pp. 983–994.
Lisy K, Langdon L, Piper A, Jefford M 2019, ‘Identifying the most prevalent unmet needs of cancer survivors in Australia: a systematic review’, Asia-Pacific Journal of Clinical Oncology, vol. 15, no. 5, pp. e68–e78.
Monterosso L, Platt V, Bulsara M, Berg M 2019, ‘Systematic review and meta-analysis of patient reported outcomes for nurse-led models of survivorship care for adult cancer patients’, Cancer Treatment Reviews Journal, no. 73, pp. 62–72.
My Health Record 2019, ‘What is My Health Record?’ Australian Government, Sydney, viewed 17 December 2019, https://www.myhealthrecord.gov.au/for-you-your-family/what-is-my-health-record.
National Cancer Survivorship Initiative (NCSI) 2015, ‘Stratified pathways of care’, National Health Service, UK, viewed 2 March 2015.
National Disability Insurance Agency 2018, ‘How the NDIS works’, NDIS, Canberra, viewed 3 June 2019, https://www.ndis.gov.au/understanding/how-ndis-works.
National Health and Medical Research Council (NHMRC) 2013, Personalised medicine and genetics, viewed 3 June 2019, https://www.nhmrc.gov.au/about-us/publications/personalised-medicine-and-genetics.
National Health and Medical Research Council (NHMRC) 2014, Talking with your patients about complementary medicine: a resource for clinicians, NHMRC, Canberra, viewed 18 February 2020, https://www.caresearch.com.au/caresearch/ClinicalPractice/PatientConsiderations/ComplementaryTherapies/tabid/1258/Default.aspx.
palliAGED 2018, ‘Needs Assessment’, Flinders University, Bedford Park, viewed 1 October 2019, https://www.palliaged.com.au/tabid/4879/Default.aspx.
Palliative Care Australia 2018, National Palliative Care Standards, 5th edn, Palliative Care Australia, Canberra, viewed 24 July 2019, http://palliativecare.org.au/wp-content/uploads/dlm_uploads/2018/11/PalliativeCare-National-Standards-2018_Nov-web.pdf.
Palliative Care Victoria 2019, ‘Spiritual care’, Palliative Care Victoria, Melbourne, viewed 22 July 2019, https://www.pallcarevic.asn.au/healthcare-professionals/about-palliative-care/care/spiritual-care/.
Queensland Health 2015, Sad news sorry business: guidelines for caring for Aboriginal and Torres Strait Islander people through death and dying, State Government of Queensland, Brisbane, viewed 22 July 2019, https://www.health.qld.gov.au/__data/assets/pdf_file/0023/151736/sorry_business.pdf.
Reese JB, Finan PH, Haythornthwaite JA, Kadan M, Regan KR, Herman JM, et al. 2014, ‘Gastrointestinal ostomies and sexual outcomes: a comparison of colorectal cancer patients by ostomy status’, Support Care Cancer, vol. 22, no. 2, pp. 461–468.
Silver JK, Baima J 2013, ‘Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes’, American Journal of Physical Medicine & Rehabilitation, vol. 92, no. 8, pp. 715–727.
Silver JK 2015, ‘Cancer prehabilitation and its role in improving health outcomes and reducing health care costs’, Seminars in Oncology Nursing, vol. 31, no. 1, pp. 1–3.
Sjoquist K, Zalcberg J 2013, ‘Clinical trials – advancing cancer care’, Cancer Forum, vol. 37, no. 1, pp. 80–88.
Smith A, Bellizzi K, Keegan T, Zebrack B, Chen V, Neale A, et al. 2013, ‘Health-related quality of life of adolescent and young adult patients with cancer in the United States: the Adolescent and Young Adult Health Outcomes and Patient Experience Study’, Journal of Clinical Oncology, vol. 31, no. 17, pp. 2136–2145.
Smith S, Case L, Waterhouse K, Pettitt N, Beddard L, Oldham J, et al. 2012, A blueprint of care for teenagers and young adults with cancer, Teenage Cancer Trust, Manchester, UK.
Steer B, Marx G, Singhal N, McJannett M, Goldstein D, Prowse R 2009, ‘Cancer in older people: a tale of two disciplines’, Internal Medicine Journal, vol. 39, pp. 771–775.
Tan SY, Turner J, Kerin-Ayres K, Butler S, Deguchi C, Khatri S, et al. 2019, ‘Health concerns of cancer survivors after primary anti-cancer treatment’, Support Cancer Care, no. 10, pp. 3739–3747.
Temel J, Greer J, Muzikansky A, Gallagher E, Admane S, Jackson V 2010, ‘Early palliative care for patients with non-metastatic non-small cell lung cancer’, New England Journal of Medicine, vol. 363, no. 8, pp. 733–742.
Vardy JL, Raymond JC, Koczwara B, Lisy K, Cohn RJ, Joske D, et al. 2019, ‘Clinical Oncology Society of Australia position statement on cancer survivorship care’, Australian Journal of General Practice, vol. 48, no. 12, pp. 833–836.
Vijayvergia N, Denlinger CS 2015, ‘Lifestyle factors in cancer survivorship: where we are and where we are headed’, Journal of Personalized Medicine, vol. 5, no. 3, pp. 243–263.
Wildiers H, Heeren P, Puts M, Topinkova E, Maryska LG, Janssen-Heijnen MLG, et al. 2014, ‘International Society of Geriatric Oncology Consensus on geriatric assessment in older patients with cancer’, Journal of Clinical Oncology, vol. 32, no. 24, pp. 2595–2603.
World Health Organization (WHO) 2018, ‘Malnutrition’, WHO, Geneva, viewed 18 July 2019, https://www.who.int/news-room/fact-sheets/detail/malnutrition.
Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. 2014, ‘Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial’, Lancet, vol. 383, no. 9930, pp. 1721–1730.







